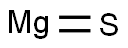MAGNESIUM SULFIDE
- CAS No.
- 12032-36-9
- Chemical Name:
- MAGNESIUM SULFIDE
- Synonyms
- MgS;Einecs 234-771-1;MAGNESIUM SULFIDE;magnesiumsulphide;magnesiumsulfide(mgs);MAGNESIUM SULFIDE 99.9%;Magnesium sulfide (MgS);MAGNESIUM SULFIDE, 99.5%;Magnesium Boride (MgB2) Sputtering Targets
- CBNumber:
- CB6706830
- Molecular Formula:
- MgS
Lewis structure

- Molecular Weight:
- 56.37
- MDL Number:
- MFCD00016207
- MOL File:
- 12032-36-9.mol
- MSDS File:
- SDS
| Melting point | >2000°C (dec.) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density | 2,84 g/cm3 | ||||||||||||||
| solubility | reacts with H2O | ||||||||||||||
| form | red-brown cubic crystals | ||||||||||||||
| color | red-brown cubic crystals, crystalline | ||||||||||||||
| Water Solubility | decomposes in H2O [HAW93] | ||||||||||||||
| Crystal Structure | NaCl type | ||||||||||||||
| crystal system | Cube | ||||||||||||||
| Space group | Fm3m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| EWG's Food Scores | 1-2 | ||||||||||||||
| FDA UNII | TWD834A2KD | ||||||||||||||
| EPA Substance Registry System | Magnesium sulfide (MgS) (12032-36-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319 |
| Precautionary statements | P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313 |
| Risk Statements | 32 |
| Safety Statements | 7/8 |
MAGNESIUM SULFIDE Chemical Properties,Uses,Production
Chemical Properties
Red-brown, crystalline solid.decomposes in water.
Chemical Properties
Magnesium Sulfide is a red-brown, crystalline solid, decomposes above 2000 C, decomposes in water.
Physical properties
MgS is a white crystalline material but is often
encountered in an impure form that is a brown noncrystalline
powder.
The chemical properties of MgS resemble those of
related ionic sulfides such as those of Na, Ba, and Ca.
Magnesium Sulfide reacts with oxygen to form the corresponding sulfate,
magnesium sulfate. MgS reacts with water to give
hydrogen sulfide and magnesium hydroxide.
Uses
Magnesium Sulfide is the source of hydrogen sulfide, laboratory reagent.
Preparation
Magnesium Sulfide
can be prepared by the direct reaction of the elements,
calcined in an inert atmosphere, at a 1:1.1 molecular
ratio:
Mg+ S+ heat→MgS
MgS also forms by the reaction of hydrogen sulfide gas with soluble magnesium salts in solution.
Aside from being a component
of some slags, MgS is a rare nonterrestial mineral
“niningerite” detected in some meteorites.
Magnesium sulfide is available commercially from
a number of suppliers.
MAGNESIUM SULFIDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| TopScience Biochemical | 00852-68527855 | info@itopbiochem.com | China Hong Kong | 902 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6312 | 58 |
| Henan Alfa Chemical Co., Ltd | +86-18339805032; +8618339805032 | alfa4@alfachem.cn | China | 13227 | 58 |
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 | sales01@sdlookchemical.com | China | 2737 | 58 |
| Alfa Chemistry | Info@alfa-chemistry.com | United States | 24072 | 58 | |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8670 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-17333973358 | sales06@hbduling.cn | China | 8054 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33338 | 58 |
12032-36-9(MAGNESIUM SULFIDE)Related Search:
1of4