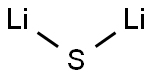
Is Lithium sulfide an ionic or covalent compound?
Dec 10,2024
Lithium sulfide is an inorganic compound with the formula Li₂S. It crystallizes in the antifluorite motif, described as the salt (Li⁺)₂S²⁻. It forms a solid yellow-white deliquescent powder. In the air, it easily hydrolyses to release hydrogen sulfide.
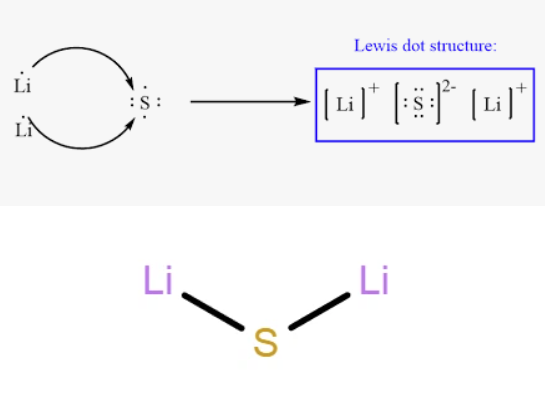
Lithium Sulfide (sulphide) is an ionic compound. In this compound, lithium atoms lose electrons to form positive ions (Li+), while sulfur gains electrons to form negative ions (S2-). These oppositely charged ions are held together by ionic bonds, which are strong electrostatic forces of attraction between the positive and negative ions.
A molecular model of lithium sulfide would depict two spheres representing lithium atoms and one larger sphere representing the sulfur atom. The atoms in lithium sulfide are indeed held together by these ionic bonds, resulting from the transfer of electrons from lithium to sulfur during chemical bonding.
- Related articles
- Related Qustion
Hydrocortisone acetate, a synthetic derivative of the natural steroid hormone hydrocortisone, is widely used in the pharmaceutical and healthcare industries.....
Apr 10,2025Chemical ReagentsBleomycin sulfate is a cytotoxic sulfur-containing polypeptide mixture of glycoproteins that can be separated by chromatography into two main fractions: bleomycin A and B.....
Dec 10,2024DrugsLithium sulfide
12136-58-2You may like
- Lithium sulfide
-

- $1000.00 / 1kg
- 2025-04-07
- CAS:12136-58-2
- Min. Order: 1kg
- Purity: 99.9%
- Supply Ability: 1T
- Lithium sulfide
-
- $0.00 / 1KG
- 2025-04-04
- CAS:12136-58-2
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1ton
- Lithium sulfide
-

- $0.00 / 1KG
- 2025-03-07
- CAS:12136-58-2
- Min. Order: 1KG
- Purity: 99.9%/99.99%
- Supply Ability: 100KG/Month