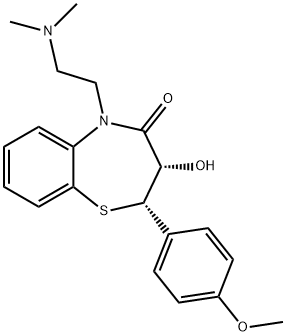
Diltiazem hydrochloride
- CAS No.
- 33286-22-5
- Chemical Name:
- Diltiazem hydrochloride
- Synonyms
- DILTIAZEM HCL;DILTHIAZEM HYDROCHLORIDE;Tiazac;Sprix;Acuvail;cardizem;herbesser;Dilacor XR;Diltiazem Hydrochlorid;(2S,cis)-3-(Acetyloxy)-5-[(2-dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one Hydrochloride
- CBNumber:
- CB7302994
- Molecular Formula:
- C22H27ClN2O4S
- Molecular Weight:
- 450.98
- MDL Number:
- MFCD00069252
- MOL File:
- 33286-22-5.mol
- MSDS File:
- SDS
| Melting point | 212-214 °C |
|---|---|
| alpha | D24 +98.3 ± 1.4° (c = 1.002 in methanol) |
| Density | 1.30 g/cm3 |
| refractive index | 118 ° (C=1, H2O) |
| Flash point | 2℃ |
| storage temp. | 2-8°C |
| solubility | Freely soluble in water, in methanol and in methylene chloride, slightly soluble in anhydrous ethanol. |
| form | Powder |
| color | White to off-white |
| PH | pH (10g/l, 25℃) : 4.3~5.3 |
| Water Solubility | soluble |
| Merck | 13,3226 |
| BRN | 4228706 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 33286-22-5(CAS DataBase Reference) |
| FDA UNII | OLH94387TE |
| Proposition 65 List | Diltiazem Hydrochloride |
| NCI Drug Dictionary | Cardizem |
| EPA Substance Registry System | 1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, hydrochloride (1:1), (2S,3S)- (33286-22-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P301+P312+P330 | |||||||||
| Hazard Codes | Xn,Xi,F | |||||||||
| Risk Statements | 22-40-36/37/38-36-20/21/22-11 | |||||||||
| Safety Statements | 36-45-36/37-26-16 | |||||||||
| RIDADR | 3249 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | DL0310000 | |||||||||
| F | 8-10 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29349990 | |||||||||
| Toxicity | LD50 in male, female mice, male, female rats (mg/kg): 61, 58, 38, 39 i.v.; 260, 280, 520, 550 s.c.; 740, 640, 560, 610 orally (Nagao, 1972) | |||||||||
| NFPA 704 |
|
Diltiazem hydrochloride price More Price(50)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR2856 | Diltiazem Hydrochloride certified reference material, pharmaceutical secondary standard | 33286-22-5 | 500MG | $218 | 2024-03-01 | Buy |
| Sigma-Aldrich | D-035 | Diltiazem hydrochloride solution 1.0?mg/mL in acetonitrile (as free base), ampule of 1?mL, certified reference material, Cerilliant? | 33286-22-5 | 1mL | $69 | 2024-03-01 | Buy |
| Sigma-Aldrich | 309866 | Diltiazem, Hydrochloride Synthetic benzothiazepine that acts as an L-type Ca | 33286-22-5 | 100mg | $96.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1205003 | Diltiazem hydrochloride United States Pharmacopeia (USP) Reference Standard | 33286-22-5 | 200mg | $436 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP899 | Diltiazem impurity standard British Pharmacopoeia (BP) Reference Standard | 33286-22-5 | 25MG | $227 | 2023-01-07 | Buy |
Diltiazem hydrochloride Chemical Properties,Uses,Production
Description
Diltiazem HCl (33286-22-5) is a?non-dihydropyridine-type blocker of L-type Ca2+ channels.1,2 Reduces Ca2+ oscillations in subcellular compartments in vascular smooth muscle cells.3?Also blocks P-type Ca2+ channels in freshly dissociated rat cerebellar Purkinje neurons.4 Clinically useful antihypertensive agent.5?Cell permeable.
Chemical Properties
Diltiazem hydrochloride is a white to off-white crystalline powder with a bitter taste. It is soluble in water, methanol, and chloroform. It has a molecular weight of 450.98. Diltiazem hydrochloride injection is a clear, colorless, sterile, nonpyrogenic solution. It has a pH range of 3.7 to 4.1.
Originator
Herbesser,TANABE SEIYAKU,Japan,1974
Uses
Peripheral Vasodilator
Uses
antihypertensive, sedative, antibacterial
Uses
Diltiazem Hydrochloride is a calcium channel blocker with vasodilating activity. Anti-anginal; anti-hypertensive; anti-arrhythmic (class IV).
Uses
An antianginal, antihypertensive. Regulates Calcium release from intracellular stores in neutrophils
Definition
ChEBI: Diltiazem hydrochloride is a hydrochloride salt resulting from the reaction of equimolar amounts of diltiazem and hydrogen chloride. A calcium-channel blocker and vasodilator, it is used in the management of angina pectoris and hypertension. It has a role as an antihypertensive agent, a vasodilator agent and a calcium channel blocker. It contains a diltiazem(1+). It is an enantiomer of an ent-diltiazem hydrochloride.
Manufacturing Process
β-Diethylaminoethyl chloride is condensed with 2-(4-methoxyphenyl)-3-
hydroxy-2,3-dihydro-1,5-benzothiazepin-4(5H)-one in a first step. Then a
mixture of 1.5 grams of 2-(4-methoxyphenyl)-3-hydroxy-5-(βdimethylaminoethyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one and 20 ml of
acetic anhydride was heated on a water bath for 5 hours. The reaction
mixture was evaporated under reduced pressure to remove acetic anhydride
and the concentrated product was poured into ice water. The resulting mixture
was made alkaline with sodium bicarbonate and extracted with chloroform.
The chloroform layer was dried and evaporated to remove the solvent. The
residue was dissolved in acetone, and an ethanol solution containing hydrogen
chloride was added thereto producing 1.53 grams of 2-(4-methoxyphenyl)3-
acetoxy-5-(β-dimethylaminoethyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one
hydrochloride having a melting point from 187° to 188°C.
The starting material is made by reacting 4-methoxybenzaldehyde with ethyl
chloroacetate; that product with sodium ethoxide; and that product with 2-
aminothiophenol.
brand name
Cardizem (Biovail); Cartia (Andrx); Dilacor (Watson); Diltzac (Apotex); Taztia (Andrx); Tiazac (Biovail).
Therapeutic Function
Coronary vasodilator
General Description
Diltiazem hydrochloride is a calcium ion cellular influx inhibitor. It was developed and introduced inJapan as a cardiovascular agent to treat angina pectoris. Itwas observed to dilate peripheral arteries and arterioles. Thedrug increases myocardial oxygen supply by relieving coronaryartery spasm and reduces myocardial oxygen demandby decreasing heart rate and reducing overload. Diltiazemhydrochloride is used in patients with variant angina. Thedrug has electrophysiological properties similar to those ofverapamil and is used in clinically similar treatment conditionsas an antiarrhythmic agent, but it is less potent.
The drug is absorbed rapidly and almost completely fromthe digestive tract. It reaches peak plasma levels within 1hour after administration in gelatin capsules. Oral formulationson the market are sustained-release preparations providingpeak plasma levels 3 to 4 hours after administration.
Biological Activity
Antihypertensive and cardioprotective agent; an inhibitor of L-type Ca 2+ channels.
Biochem/physiol Actions
Product does not compete with ATP.
Clinical Use
Calcium-channel blocker:
Prophylaxis and treatment of angina
Hypertension
Metabolism
Diltiazem is almost completely absorbed from the
gastrointestinal tract after oral doses, but undergoes
extensive first-pass hepatic metabolism resulting in a
bioavailability of about 40%. It is extensively metabolised
in the liver, mainly by the cytochrome P450 isoenzyme
CYP3A4; one of the metabolites, desacetyldiltiazem,
has been reported to have 25-50% of the activity of the
parent compound
About 2-4% of a dose is excreted in urine as unchanged
diltiazem with the remainder excreted as metabolites in
bile and urine.
storage
Store at RT
Mode of action
Diltiazem Hydrochloride is a benzothiazepine calcium channel blocking agent. Diltiazem hydrochloride inhibits the transmembrane influx of extracellular calcium ions into select myocardial and vascular smooth muscle cells, causing dilatation of coronary and systemic arteries and decreasing myocardial contractility. Because of its vasodilatory activity, this agent has been shown to improve the microcirculation in some tumors, thereby potentially improving the delivery of antineoplastic agents to tumor cells.
References
1) Kraus?et al.?(1998),?Molecular mechanism of diltiazem interaction with L-type Ca2+ channels; J. Biol. Chem.,?273?27205 2) Godfraind?et al. (1986),?Calcium antagonism and calcium entry blockade; Pharmacol. Rev.,?38?321 3) Fedoryak?et al.?(2004),?Spontaneous Ca2+ oscillations in subcellular compartments of vascular smooth muscle cells rely on different Ca2+ pools; Cell Res.,?14?379 4) Ishibashi?et al. (1995),?Block of P-type Ca2+ channels in freshly dissociated rat cerebellar Purkinje neurons by diltiazem and verapamil; Brain Res.,?695?88 5) Chaffman and Bogden (1985),?Diltiazem. A review of its pharmacological properties and therapeutic efficacy; Drugs,?29?387
Diltiazem hydrochloride Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Jinhuan Chemical CO.,LTD. | +86-21-52210535 +8613162045318 | sales@jinhchem.com | China | 331 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 | bess@weibangbio.com | China | 18154 | 58 |
| Suzhou Sanyi Polymer Chemical Technology Co., Ltd. | +8615571922873 | 15571922873@163.com | China | 102 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Guangzhou Tengyue Chemical Co., Ltd. | +86-86-18148706580 +8618826483838 | evan@tyvovo.com | China | 148 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 973 | 58 |
| Shaanxi Franta Biotechnology Co., Ltd | +86-13082019107 +86-13082019107 | admin@flanderff.com | China | 206 | 58 |
| Hebei Mojin Biotechnology Co.,Ltd | +86-15028179902 | angelia@hbmojin.com | China | 1177 | 58 |
| Hebei Ganmiao New material Technology Co., LTD | +86-17332992504 +86-17332992504 | sales8@hbganmiao.com | China | 299 | 58 |
| Binzhou Lista Trading Limited | sallychris2021@gmail.com | China | 74 | 58 |
View Lastest Price from Diltiazem hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Diltiazem hydrochloride
33286-22-5
|
US $46.00 / mg | 99.65% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-19 | Diltiazem hydrochloride
33286-22-5
|
US $46.00 / mg | 99.65% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-18 | diltiazem hydrochloride
33286-22-5
|
US $20.00 / kg | 1kg | 99.9% | 10000 | Hebei Miaoyin Technology Co.,Ltd |
-

- Diltiazem hydrochloride
33286-22-5
- US $46.00 / mg
- 99.65%
- TargetMol Chemicals Inc.
-

- Diltiazem hydrochloride
33286-22-5
- US $46.00 / mg
- 99.65%
- TargetMol Chemicals Inc.
-

- diltiazem hydrochloride
33286-22-5
- US $20.00 / kg
- 99.9%
- Hebei Miaoyin Technology Co.,Ltd
33286-22-5(Diltiazem hydrochloride)Related Search:
1of4