Cyclopentane
- CAS No.
- 287-92-3
- Chemical Name:
- Cyclopentane
- Synonyms
- PENTAMETHYLENE;Cyclopentan;opentane;Cyclopcntan;CYCLOPENTANE;Cyclopentane 5;BLENDED PENTANE;Cyclopentane >Cyclopentane,95+%;(Cyclopentane) CAS:
- CBNumber:
- CB7363777
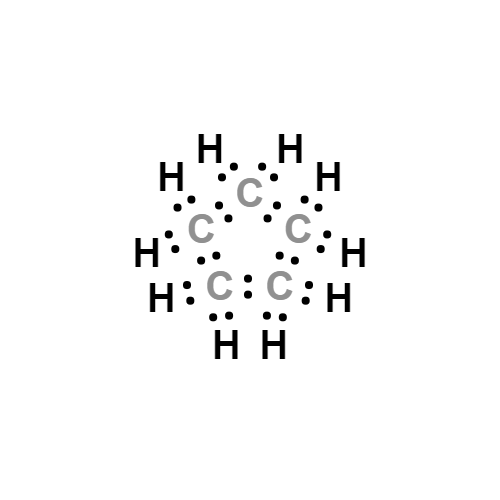
- Molecular Formula:
- C5H10
Lewis structure
- Molecular Weight:
- 70.13
- MDL Number:
- MFCD00001356
- MOL File:
- 287-92-3.mol
- MSDS File:
- SDS
| Melting point | -94 °C (lit.) |
|---|---|
| Boiling point | 50 °C (lit.) |
| Density | 0.751 g/mL at 25 °C (lit.) |
| vapor density | ~2 (vs air) |
| vapor pressure | 18.93 psi ( 55 °C) |
| refractive index |
n |
| Flash point | −35 °F |
| storage temp. | Store below +30°C. |
| solubility | 0.156g/l insoluble |
| form | Powder |
| color | White |
| Odor | Like gasoline; mild, sweet. |
| explosive limit | 1.5-8.7%(V) |
| Water Solubility | Miscible with ethanol, ether and acetone. Slightly miscible with water. |
| λmax |
λ: 198 nm Amax: 1.0 λ: 210 nm Amax: 0.50 λ: 220 nm Amax: 0.10 λ: 240-400 nm Amax: 0.01 |
| Merck | 14,2741 |
| BRN | 1900195 |
| Henry's Law Constant | 0.164, 0.240, and 0.300 at 27.9, 35.8, and 45.0 °C, respectively (dynamic headspace, Hansen et al., 1995) |
| Exposure limits | TLV-TWA 600 ppm (~1720 mg/m3) (ACGIH). |
| Dielectric constant | 1.9(20℃) |
| Stability | Stable. Highly flammable. Note low flash point and wide explosion limits. Incompatible with strong oxidizing agents. Floats on water, so water is of limited value in putting out fires involving this material. |
| LogP | 3 at 20℃ |
| CAS DataBase Reference | 287-92-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | T86PB90RNU |
| NIST Chemistry Reference | Cyclopentane(287-92-3) |
| EPA Substance Registry System | Cyclopentane (287-92-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS02 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H412 | |||||||||
| Precautionary statements | P210-P273 | |||||||||
| Hazard Codes | F | |||||||||
| Risk Statements | 11-52/53 | |||||||||
| Safety Statements | 9-16-29-33-61 | |||||||||
| RIDADR | UN 1146 3/PG 2 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 600 ppm (1720 mg/m3) | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | GY2390000 | |||||||||
| Autoignition Temperature | 682 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2902 19 00 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LC (2 hr in air) in mice: 110 mg/l (Lazarew) | |||||||||
| NFPA 704 |
|
Cyclopentane price More Price(25)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.18770 | Cyclopentane for synthesis | 287-92-3 | 100mL | $28.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.18770 | Cyclopentane for synthesis | 287-92-3 | 500mL | $109 | 2024-03-01 | Buy |
| Sigma-Aldrich | CX2414 | Cyclopentane OmniSolv? | 287-92-3 | 4L | $503 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.18770 | Cyclopentane for synthesis | 287-92-3 | 2.5L | $334 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.18769 | Cyclopentane for synthesis | 287-92-3 | 250ML | $75.5 | 2024-03-01 | Buy |
Cyclopentane Chemical Properties,Uses,Production
Physical and chemical properties
Cyclopentane, also known as "pentamethylene", is a kind of cycloalkane with the formula of C5H10. It has a molecular weight of 70.13. It exists as a kind of flammable liquid. It has a melting point-94.4 °C, boiling point of 49.3 °C, relative density of 0.7460 and the refractive index of 1.4068. It is soluble in alcohol, ether and hydrocarbons and is not soluble in water. Cyclopentane is not a planar ring and has two conformations: envelope conformations and semi-chair conformations. The carbon-carbon-carbon bond angle is close to 109 ° 28 ' with the molecular tension not big and the ring being relatively stable. It has a similar chemical property as alkanes. The lethal concentration in the air for the rats was 3.8 × 10-2. It exhibits red yellow color when having reaction with fuming sulfuric acid while generating nitro cyclopentane and glutaric acid through reaction with nitric acid. Method: it can be obtained from the petroleum ether distillate, through high-pressure cracking on the cyclohexane in the presence of aluminum or catalytic hydrogenation of cyclopentene and cyclopentadiene. Purposes: mainly used as a solvent.
Figure 1 the cyclopentane structure.
Cycloalkane
Cycloalkanes are saturated hydrocarbons in which the carbon atoms in the molecule are arranged in a ring and a sufficient number of hydrogen atoms are combined. Cycloalkanes presented in petroleum are mainly cyclopentane and cyclohexane.
Cycloalkanes have a higher melting point, boiling point, and relative density than the corresponding straight-chain alkanes. We can use the naphthenic aromatic crude oil to produce high-octane straight-run gasoline with its anti-explosion being better than normal paraffin. Low sulfur-containing paraffin naphthenic crude oil, is not only easy to process, but also an excellent raw material for the production of advanced lubricants. Petroleum containing relative many polycyclic long side chain naphthenic compounds is an ideal material for high-quality lubricants.
At room temperature and atmospheric pressure, cycloalkane containing four or less carbon atoms is in the gas form with those contain more than four carbons existing in the liquid form. The cyclopropane and cyclobutane appear as gas, cyclopentane to cycloundecane appears as liquid; cyclododecane and above appears as solid.
The chemical nature of the cycloalkane is related to the number of carbon atoms forming the ring. It is referred three-membered ring and four-membered ring, as small ring; the five-to-seven-membered ring as normal ring; the eight-eleven ring as normal ring; the twelve-membered ring and above as large ring. The nuclei lines of carbon nuclei in small rings are not consistent with the axis of bonding orbital. In cyclopropane, the ring formed by the nucleus lines of carbon atoms is an equilateral triangle with each angle being 60° while the angle of the sp3 hybrid orbital axis of carbon-carbon single bonds formed by each carbon 104° (see figure 2 below). Therefore, the orbit has failed to achieve the greatest degree of overlap, causing a large angle tension. Cyclobutane also has angular tension, but being smaller. This leads to the poor stability of the small ring, causing its similar chemical property to olefins that can have ring-opening addition reaction with many reagents. Other ring has less of no angular tension. Cycloalkane and alkanes have similar chemical properties, less prone to have ring-opening reaction such as having reaction with hydrogen. Cyclohexane and higher cycloalkane is more difficult to undergo hydrogenation.
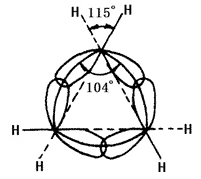
Figure 2 is a schematic representation of sp3 hybridization orbital overlap in cyclopropane.
Cyclopropane (at room temperature) and cyclobutane (at the heating conditions) can have addition reaction with halogen and hydrogen halide.
The open-loop occurs between the two atoms connecting the most and least numbers of hydrogen. The addition satisfies the Markovian rule. While the normal ring, under the stimulation of the light or heat can have substitution reaction with the halogen.
At room temperature, cycloalkane can’t be oxidized by potassium permanganate.
Cyclopentane, cyclohexane and its alkyl substituted products are presented in certain petroleum oils. Cycloalkanes may also be synthesized by suitable methods, such as dihaloalkane cyclization and hydrogenation of aromatic hydrocarbons.
This information was edited by Xiaonan from Chemicalbook (2015-08-17).
Dangerous situation
Ingestion and inhalation are moderately toxic. (2) Being flammable with greater risk of combustion. The allowable concentration in air is 600ppm (1720mg/m3) in the United States.
Harmful effects and symptoms of poisoning
Inhalation of high concentrations of cyclopentane can cause central nervous system inhibition, although its acute toxicity is low. Symptoms of acute exposure include excitement at first, followed by the emergence of balance disorders, and even stupor, coma. There are rarely cases of death due to respiratory failure. It has been reported that animals fed with this goods can get severe diarrhea, leading to heart, lung and liver vascular collapse and brain degeneration.
Protective measures
It can be used for improving the production equipment. Use skin protective creams or gloves to protect the skin.
Medical care
Upon regular physical examination, pay attention to the potential irritation effect of skin and respiratory tract as well as any complications of kidney and liver.
Transportation requirements
Grade I flammable liquid. Code of Hazard Regulations: 61013. The container shall be marked with a "flammable liquid" mark on transport.
Fire extinguishing agent
See “cyclohexane”.
Recommended waste disposal methods
Incineration;
Chemical properties
It appears as colorless liquid with a melting point of-93.9 °C, the boiling point of 49.26 ° C, the relative density of 0.7460 (20/4 ° C), the refractive index of 1.4068 and the flash point of-37 °C. It is miscible with alcohol, ether and other organic solvents, being not be easy to be dissolved in water.
Uses
(1) It can be used as a solvent for solution polymerization of polyisoprene rubber and cellulose ether. It can be used as a substitute for Freon as insulation materials in refrigerators and freezers as well as foaming agents for other hard PU foams, and chromatographic analysis standards.
(2) Used as a standard substance for chromatographic analysis, solvents, engine fuels, azeotropic distillation agents.
Production method
Cyclopentane is a component of the petroleum ether in the 30-60 °C boiling point range with the content being generally 5%-10%. Apply atmospheric distillation; at a 60: 1 reflux ratio and carry out at an 8m height tower; first distill out the isopentane and n-pentane; continue fractionation to obtain a cyclopentane with purity being over 98%. Cyclopentane can also be obtained through cyclopentanone reduction or cyclopentadiene catalytic hydrogenation.
Hazards & Safety Information
Category Flammable liquids
Toxicity grading: Low toxicity
Acute toxicity: oral-rat LD50: 11400 mg/kg; oral-mouse LD50: 12800 mg/kg
Hazardous property of explosives: being explosive upon mixed with air
Flammability and dangerous situations: being flammable in case of fire, high temperature and oxidant with combustion releasing irritant smoke
Storage characteristics: Storehouse: ventilated, low temperature and dry; Store it with oxidant separately
Extinguishing agent: Dry powder, carbon dioxide, foam, 1211 extinguishing agent
Occupational standard: TWA 1720 mg/m3; STEL 2150 mg/kg
Description
Cyclopentane is a colorless liquid. Molecularweight=70.15; Specific gravity=(H2O:1)=0.75; Boilingpoint=49℃; Freezing/Melting point=2 93.6℃; Vaporpressure=400 mmHg at 31℃; Relative vapor density (air-5 1)=2.42: Flash point=37.2℃; Autoignitiontemperature=361℃. Explosive limits in air: LEL=1.1%;UEL=8.7%. Hazard Identification (based on NFPA-704 MRating System): Health 1, Flammability 3, Reactivity 0.Insoluble in water
Chemical Properties
Cyclopentane is a colourless flammable acyclic hydrocarbon liquid with a petrol-like smell. It is a widely used component in preparing products like analgesics, insecticides, sedatives, antitumor agents and also finds application in pharmaceutical industry.
Physical properties
Colorless, mobile, flammable liquid with an odor resembling cyclohexane.
Uses
As a laboratory reagent; in the manufacture of pharmaceuticals; found in solvents and in petroleum ether; propellant pressurizing agent.
Uses
Cyclopentane is a petroleum product. Itis formed from high-temperature catalyticcracking of cyclohexane or by reduction ofcyclopentadiene. It occurs in petroleum etherfractions and in many commercial solvents.It is used as a solvent for paint, in extractionsof wax and fat, and in the shoe industry.
Uses
Cyclopentane is used as green blowing agent and involved in the production of polyurethane insulating foam, which is used in refrigerators, freezers, water heaters, construction panels, insulated pipes and roofs. As a lubricant, it finds applications in computer hard drives and outer space equipment due to its low volatile nature. It is widely useful in the preparation of resin, adhesives and pharmaceutical intermediate. It is an additive in gasoline. Since it is a halogen free compound and has zero-ozone depletion potential, it replaces the conventionally used chloro fluoro carbon (CFC) in refrigeration and thermal insulation.
Production Methods
Cyclopentane occurs in petroleum ether fractions and is prepared by cracking cyclohexane in the presence of alumina at high temperature and pressure or by reduction of cyclopentadiene.
Definition
ChEBI: A cycloalkane that consists of five carbons each bonded with two hydrogens above and below the plane. The parent of the class of cyclopentanes.
General Description
A clear colorless liquid with a petroleum-like odor. Flash point of -35°F. Less dense than water and insoluble in water. Vapors are heavier than air.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
CYCLOPENTANE is incompatible with strong oxidizing agents such as chlorine, bromine, fluorine. .
Health Hazard
Cyclopentane is a low-acute toxicant. Itsexposure at high concentrations may producedepression of the central nervous system withsymptoms of excitability, loss of equilibrium,stupor, and coma. Respiratory failure may occur in rats from 30–60 minutes’ exposureto 100,000–120,000 ppm in air. It is anirritant to the upper respiratory tract, skin,and eyes. No information is available inthe literature on the chronic effects fromprolonged exposure to cyclopentane.
Fire Hazard
Behavior in Fire: Containers may explode.
Flammability and Explosibility
Highly flammable
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Mildly toxic by ingestion and inhalation. High concentrations have narcotic action. A very dangerous fire hazard when exposed to heat or flame; can react with oxidizers. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes.
Potential Exposure
Cyclopentane is used as a solvent.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get mediing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Do not induce vomiting, guard against aspiration. Medical observation is recommended for 24-48 hafter breathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
Source
Component of high octane gasoline (quoted, Verschueren, 1983). Harley et al. (2000)
analyzed the headspace vapors of three grades of unleaded gasoline where ethanol was added to replace methyl tert-butyl ether. Cyclopentane was detected at an identical concentration of 1.4 wt
% in the headspace vapors for regular, mid-, and premium grades.
Schauer et al. (1999) reported cyclopentane in a diesel-powered medium-duty truck exhaust at
an emission rate of 410 μg/km.
California Phase II reformulated gasoline contained cyclopentane at a concentration of 4.11
g/kg. Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without
catalytic converters were 0.78 and 85.4 mg/km, respectively (Schauer et al., 2002).
Environmental Fate
Biological. Cyclopentane may be oxidized by microbes to cyclopentanol, which may oxidize to
cyclopentanone (Dugan, 1972).
Photolytic. The following rate constants were reported for the reaction of octane and OH
radicals in the atmosphere: 3.7 x 10-12 cm3/molecule?sec at 300 K (Hendry and Kenley, 1979); 5.40
x 10-12 cm3/molecule?sec (Atkinson, 1979); 4.83 x 10-12 cm3/molecule?sec at 298 K (DeMore and
Bayes, 1999); 6.20 x 10-12, 5.24 x 10-12, and 4.43 x 10-12 cm3/molecule?sec at 298, 299, and 300 K,
respectively (Atkinson, 1985), 5.16 x 10-12 cm3/molecule?sec at 298 K (Atkinson, 1990), and 5.02
x 10-12 cm3/mol·sec at 295 K (Droege and Tilly, 1987).
Chemical/Physical. Cyclopentane will not hydrolyze because it has no hydrolyzable functional
group. Complete combustion in air yields carbon dioxide and water.
At elevated temperatures, rupture of the ring occurs forming ethylene and presumably allene
and hydrogen (Rice and Murphy, 1942).
storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with cyclopentane you should be trainedon its proper handling and storage. Before entering confinedspace where cyclopentane may be present, check to makesure that an explosive concentration does not exist. Store intightly closed containers in a cool well-ventilated area awayfrom strong oxidizers (such as chlorine, bromine, and fluorine). Use only nonsparking tools and equipment, especiallywhen opening and closing containers of cyclopentane.Metal containers involving the transfer of this chemicalshould be grounded and bonded. Where possible, automatically pump liquid from drums or other storage containers toprocess containers. Drums must be equipped with selfclosing valves, pressure vacuum bungs, and flame arresters.Use only nonsparking tools and equipment, especially whenopening and closing containers of this chemical. Sources ofignition, such as smoking and open flames, are prohibitedwhere this chemical is used, handled, or stored in a mannerthat could create a potential fire or explosion hazard.Wherever this chemical is used, handled, manufactured, orstored, use explosion-proof electrical equipment andfittings.
Shipping
UN1146 Cyclopentane, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
Free it from cyclopentene by two passages through a column of carefully dried and degassed activated silica gel. It occurs in petroleum and is HIGHLY FLAMMABLE. [NMR: Christl Chem Ber 108 2781 1975, Whitesides et al. 41 2882 1976, Beilstein 5 III 10, 5 IV 4.]
Incompatibilities
May form explosive mixture with air. May accumulate static electrical charges, and may cause ignition of its vapors. Contact with strong oxidizers may cause fire and explosion.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera- tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Cyclopentane Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20286 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 | catherine@yjchem.com.cn | China | 985 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29881 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2930 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
Related articles
- Uses and health risk of Cyclopentane
- ?Cyclopentane is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS number 287-92-3, consisting of a....
- Apr 14,2022
View Lastest Price from Cyclopentane manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-10-30 | Cyclopentane
287-92-3
|
US $100.00 / Kg/Drum | 1Kg/Drum | 99%min | 200TON | Hebei Weibang Biotechnology Co., Ltd | |
 |
2024-10-11 | Cyclopentane
287-92-3
|
US $10.50 / KG | 1KG | 24% | 10 ton | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-05-01 | Cyclopentane.
287-92-3
|
US $1100.00-710.00 / T | 1T | 98% | 20T | Yujiang Chemical (Shandong) Co.,Ltd. |
-

- Cyclopentane
287-92-3
- US $100.00 / Kg/Drum
- 99%min
- Hebei Weibang Biotechnology Co., Ltd
-

- Cyclopentane
287-92-3
- US $10.50 / KG
- 24%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- Cyclopentane.
287-92-3
- US $1100.00-710.00 / T
- 98%
- Yujiang Chemical (Shandong) Co.,Ltd.
287-92-3(Cyclopentane)Related Search:
1of4