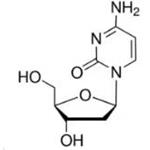
2'-Deoxycytidine
- CAS No.
- 951-77-9
- Chemical Name:
- 2'-Deoxycytidine
- Synonyms
- DC;deoxycytidine;4-AMino-1-((2R,4S,5R)-4-hydroxy-5-(hydroxyMethyl)tetrahydrofuran-2-yl)pyriMidin-2(1H)-one;dCyd;2-Deoxycystidine;Cytosine deoxyribonucleoside;4-amino-1-[4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-pyrimidin-2-one;2'-DC;2'-dC;2&apos
- CBNumber:
- CB7452978
- Molecular Formula:
- C9H13N3O4
- Molecular Weight:
- 227.22
- MDL Number:
- MFCD00006547
- MOL File:
- 951-77-9.mol
- MSDS File:
- SDS
| Melting point | 209-211 °C(lit.) |
|---|---|
| Boiling point | 368.93°C (rough estimate) |
| Density | 1.3171 (rough estimate) |
| refractive index | 59 ° (C=1, H2O) |
| storage temp. | -20°C |
| solubility | H2O: 50 mg/mL, clear, colorless |
| form | Crystalline Powder |
| pka | 14.03±0.60(Predicted) |
| color | White |
| PH | 4.3 |
| Water Solubility | Soluble in water, DMSO. |
| λmax | 280 (pH 1);271 (pH 7) |
| BRN | 87567 |
| InChI | InChI=1S/C9H13N3O4/c10-7-1-2-12(9(15)11-7)8-3-5(14)6(4-13)16-8/h1-2,5-6,8,13-14H,3-4H2,(H2,10,11,15)/t5-,6+,8+/m0/s1 |
| InChIKey | CKTSBUTUHBMZGZ-SHYZEUOFSA-N |
| SMILES | OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O |
| CAS DataBase Reference | 951-77-9(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | deoxycytidine |
| FDA UNII | 0W860991D6 |
| NIST Chemistry Reference | Deoxycytidine(951-77-9) |
| EPA Substance Registry System | Cytidine, 2'-deoxy- (951-77-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P280-P302+P352-P304+P340 | |||||||||
| Safety Statements | 24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | HA3800000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29349990 | |||||||||
| NFPA 704 |
|
2'-Deoxycytidine price More Price(41)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | D3897 | 2′-Deoxycytidine ≥99% (HPLC) | 951-77-9 | 1g | $140 | 2024-03-01 | Buy |
| Sigma-Aldrich | D3897 | 2′-Deoxycytidine ≥99% (HPLC) | 951-77-9 | 5g | $482 | 2024-03-01 | Buy |
| TCI Chemical | D3583 | 2'-Deoxycytidine >98.0%(HPLC)(T) | 951-77-9 | 1g | $33 | 2024-03-01 | Buy |
| TCI Chemical | D3583 | 2'-Deoxycytidine >98.0%(HPLC)(T) | 951-77-9 | 5g | $99 | 2024-03-01 | Buy |
| Alfa Aesar | J63062 | 2'-Deoxycytidine | 951-77-9 | 1g | $28.65 | 2024-03-01 | Buy |
2'-Deoxycytidine Chemical Properties,Uses,Production
Description
2'-Deoxycytidine is a deoxyribonucleotide that is used in the synthesis of DNA. 2'-Deoxycytidine has been shown to inhibit the kinase activity of IL-2 receptor and Toll-like receptor, which are proteins that regulate the immune response. 2'-Deoxycytidine also inhibits DNA polymerase activity and thermal expansion, which may make it a good candidate as an anticancer drug.
Chemical Properties
White crystalline powder
Uses
2'-Deoxycytidine (deoxyC) is one of the deoxynucleosides which after phosphorylation to dCTP is used to synthesis DNA via various DNA polymerases or reverse transcriptases. 2'-Deoxycytidine (deoxyC) is the substrate for deoxycytidine deaminase (EC 3.5.4.14) which converts it into 2?-deoxyuridine. 2?-Deoxycytidine is phosphorylated to the nucleotide dCMP by the enzyme deoxycytidine kinase (DCK).
Uses
A deoxyribonucleoside
Definition
ChEBI: A pyrimidine 2'-deoxyribonucleoside having cytosine as the nucleobase.
General Description
2′-Deoxycytidine (deoxyC) is one of the deoxynucleosides containing cytosine as the nucleobase. It is found in the blood, feces, and urine.
Biological Activity
2-deoxycytidine is a cytidine analog [1].2-deoxycytidine prevents dna methylation by incorporating itself into newly synthesizing dna strand. 2-deoxycytidine also binds to dna methyltransferase irreversibly and hinders its activity. thus, 2-deoxycytidine was approved as the most efective demethylating agent for the treatment of cancer [1].2-deoxycytidine at clinically achievable and nontoxic concentrations (≥ 100 μmol/l) protected normal bone marrow progenitor cells against the inhibitory effects of co-administered, high concentrations of 3’-azido-3’-deoxythymidine (azt) (≥ 10 μmol/l). in normal bone marrow mononuclear cells (bmmc), 2-deoxycytidine also significantly corrected azt-mediated depletion of intracellular thymidine triphosphate and 2-deoxycytidine triphosphate levels. furthermore, 2-deoxycytidine reduced the intracellular accumulation of azt triphosphate and its dna incorporation in bmmc [2].in a rat model of myocardial infarction induced by ligating left anterior descending coronary artery, human umbilical cord mesenchymal stem cells treated with 2-deoxycytidine (5, 10, 20 and 40 μm) before transplantation to the left ventricular wall immediately after ligation significantly improved the cardiac systolic and diastolic functions, and pumping ability. fibrotic area and left ventricular wall thickness were also significantly improved [1].[1]. ali s r, ahmad w, naeem n, et al. small molecule 2'-deoxycytidine differentiates human umbilical cord-derived mscs into cardiac progenitors in vitro and their in vivo xeno-transplantation improves cardiac function. molecular and cellular biochemistry, 2020, 470(1-2): 99-113.[2]. bhalla k, birkhofer m, li g r, et al. 2'-deoxycytidine protects normal human bone marrow progenitor cells in vitro against the cytotoxicity of 3'-azido-3'-deoxythymidine with preservation of antiretroviral activity. blood, 1989, 74(6): 1923-1928.
Biochem/physiol Actions
2′-Deoxycytidine (deoxyC) forms dCTP upon phosphorylation which is used to synthesis DNA via various DNA polymerases or reverse transcriptases. DeoxyC is the substrate for deoxycytidine deaminase (EC 3.5.4.14) which converts it into 2′-deoxyuridine. DeoxyC is phosphorylated to the nucleotide dCMP by the enzyme deoxycytidine kinase (DCK). DeoxyC serves as a potential head and neck cancer marker.
Cytotoxicity
JC-1 staining was performed to determine the cytotoxic effect of 2-DC on UC-MSCs. UC-MSCs were cultured in complete DMEM and treated with the different concentrations (10, 20, 40, 60, 80, and 100 μM) of 2-DC for 24 h. Unlabeled MSCs were used as a negative control, while MSCs labelled only with JC-1 stain were used for calibrating the instrument and optimizing the protocol. Cells were harvested, washed, and stained with JC-1 dye for 15 min at 37 °C. Cells were washed with PBS and resuspended in PBS. Analysis was performed through fow cytometry. Data was acquired and analyzed using BD CellQuest pro software. UC-MSCs treated with different concentrations of 2-DC and stained with JC-1 dye showed that 2-DC did not have any significant cytotoxic effect at any of these concentrations[1].
Safety Profile
Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Purification Methods
Purify 2'-deoxycytidine by recrystallisation from MeOH/Et2O or EtOH and dry it in air. [NMR: Miles J Am Chem Soc 85 1007 1963, UV: Fox & Shugar Biochim Biophys Acta 9 369 1952.] The hydrochloride crystallises from H2O/EtOH and has m 174o(dec, 169-173o). [Walker & Butler Can J Chem 34 1168 1956.] The picrate has m 208o(dec). [Fox et al. J Am Chem Soc 83 4066 1961, Beilstein 25 III/IV 3662.]
Structure and conformation
2'-Deoxycytidine monohydrate (dCMP) is a nucleoside phosphate in being comprised of a deoxyribonucleoside and one phosphate group. It has a deoxyribose as its sugar constituent with one phosphate group attached. Its nucleoside contains a pyrimidine base, i.e., a cytosine attached to the deoxyribose sugar. It has only one phosphate group attached to the nucleoside. Its conjugate acid form is deoxycytidylic acid, whereas its conjugate base form is deoxycytidylate. dCMP, instead of having a hydroxyl group on the 2′ carbon of the sugar component as it is in Cytidine monophosphate (CMP), has it reduced to a hydrogen atom (thus, deoxy- in its name). dCMP is one of the monomeric units that constitute DNA, whereas CMP is one of the monomeric units that make up RNA.
References
[1] Syeda Roohina Ali. “Small molecule 2’-deoxycytidine differentiates human umbilical cord-derived MSCs into cardiac progenitors in vitro and their in vivo xeno-transplantation improves cardiac function.” Molecular and Cellular Biochemistry 470 1–2 (2020): 99–113.
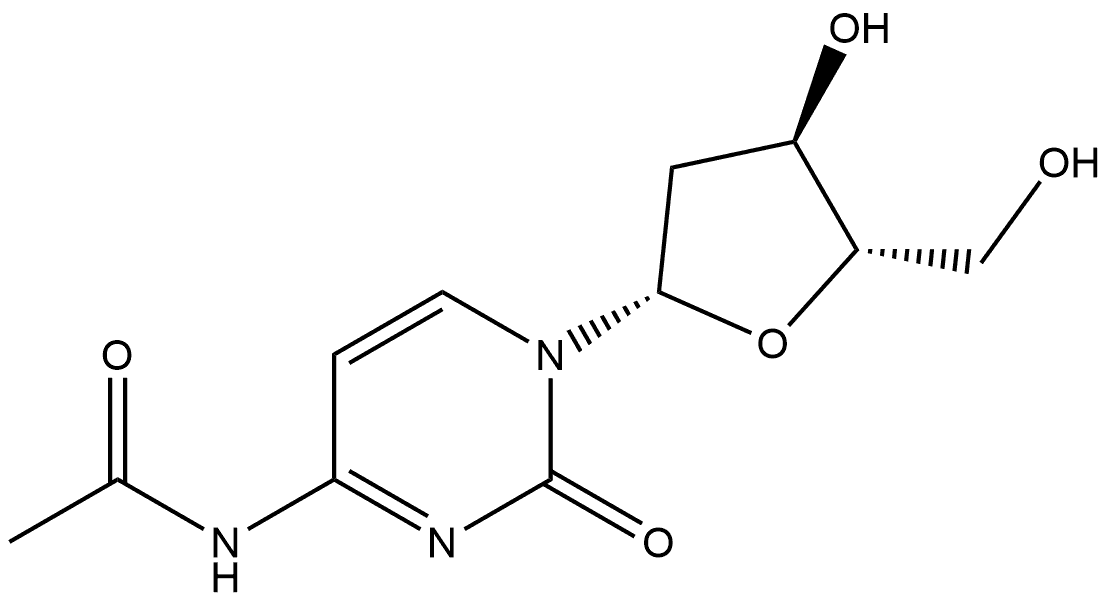
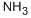
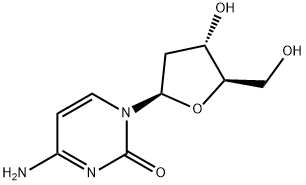
2'-Deoxycytidine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Yunbio Tech Co.,Ltd. | +86-010-60605551 +86-18046518538 | yunbiochem@126.com | China | 321 | 58 |
| Chemtour Biotech Co., Ltd | +8617327281506 | market@chemtour.com | China | 1460 | 58 |
| SHANGHAI NOVRIBO BIOTECH | 17821030819 | June.Liu@Desano.com | China | 73 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615350571055 | Sibel@weibangbio.com | China | 6087 | 58 |
| Xinxiang Aurora Biotechnology Co.,Ltd. | +86-0086-0373-3088722 +8618637352520 | info@aurora-biotech.com | China | 91 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12835 | 58 |
| HangZhou RunYan Pharma Technology Co.,LTD. | +8618882027439 | sales2@runyanpharma.com | China | 302 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18754 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21638 | 55 |
Related articles
- What are 2'-Deoxycytidine and 5-aza-2′-deoxycytidine?
- 2'-Deoxycytidine is a pyrimidine 2'-deoxyribonucleoside with cytosine as the nucleobase and the ribose lacking a hydroxyl grou....
- Nov 5,2024
View Lastest Price from 2'-Deoxycytidine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-07 | 2′-Deoxycytidine
951-77-9
|
US $0.00-0.00 / MG | 10MG | 99% HPLC | 10000 | HangZhou RunYan Pharma Technology Co.,LTD. | |
 |
2024-11-04 | 2'-Deoxycytidine
951-77-9
|
US $0.00 / Kg/Bag | 1KG | 99%min | 50KGS | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-08-08 | 2′-Deoxycytidine
951-77-9
|
US $0.00 / g | 1g | ≥99% | 100kg | Hefei Huana Biomedical Technology Co.,Ltd. |
-

- 2′-Deoxycytidine
951-77-9
- US $0.00-0.00 / MG
- 99% HPLC
- HangZhou RunYan Pharma Technology Co.,LTD.
-

- 2'-Deoxycytidine
951-77-9
- US $0.00 / Kg/Bag
- 99%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- 2′-Deoxycytidine
951-77-9
- US $0.00 / g
- ≥99%
- Hefei Huana Biomedical Technology Co.,Ltd.





