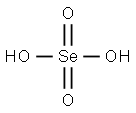
Selenic acid
- CAS No.
- 7783-08-6
- Chemical Name:
- Selenic acid
- Synonyms
- SELENIC ACID;SELENIC ACID AR;SELENIC ACID 94%;Selenocystathionine;selenic acid solution;Selenic Acid aq. Soln.;Selenicacid,40%solution;Selenicacid,40%aq.soln.;Selenic acid solution, 40 wt. % in water;Selenic acid, 99.95% metals basis, 40 wt. % solution in water
- CBNumber:
- CB7854327
- Molecular Formula:
- H2O4Se
Lewis structure
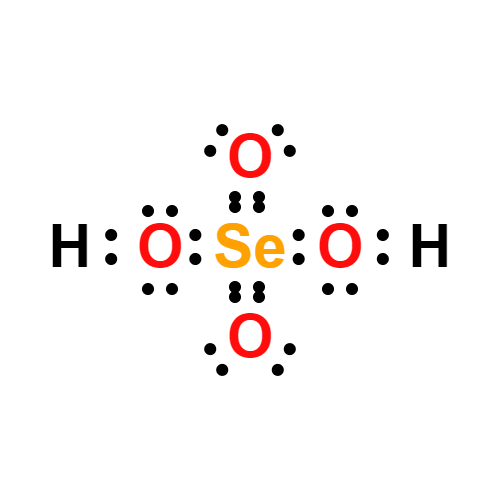
- Molecular Weight:
- 144.97
- MDL Number:
- MFCD00012191
- MOL File:
- 7783-08-6.mol
| Melting point | 58°C |
|---|---|
| Boiling point | 260°C |
| Density | 1.407 g/mL at 25 °C |
| refractive index | n20/D 1.5174(lit.) |
| Flash point | >230 °F |
| solubility | Miscible with sulfuric acid. Immiscible with ammonia. |
| form | Liquid |
| pka | 4.19±0.10(Predicted) |
| color | Colorless |
| PH | 2.74(1 mM solution);1.83(10 mM solution);0.97(100 mM solution) |
| Water Solubility | g/100g H2O: 426 (0°C), 567 (20°C), 1328 (30°C) [LAN05]; decomposed by alcohol [HAW93] |
| Merck | 14,8429 |
| Exposure limits |
ACGIH: TWA 0.2 mg/m3 NIOSH: IDLH 1 mg/m3; TWA 0.2 mg/m3 |
| Stability | Stable, but decomposes on heating. Non-flammable. Incompatible with metals, combustible materials. Hygroscopic. |
| CAS DataBase Reference | 7783-08-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | HV0Y51NC4J |
| EPA Substance Registry System | Selenic acid (7783-08-6) |
| UNSPSC Code | 12352300 |
| NACRES | NA.23 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS05,GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H331-H315-H318-H373-H410 | |||||||||
| Precautionary statements | P273-P280-P301+P310+P330-P304+P340+P311-P305+P351+P338+P310-P314 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 23/24/25-34-50/53-33-23/25-41-38 | |||||||||
| Safety Statements | 23-26-36/37/39-45-61-60-28-20/21-16 | |||||||||
| RIDADR | UN 3264 8/PG 1 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | VS6575000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 2811198090 | |||||||||
| Hazardous Substances Data | 7783-08-6(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
Selenic acid price More Price(10)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 481513 | Selenic acid solution 40 wt. % in H2O, 99.95% trace metals basis | 7783-08-6 | 25ml | $83.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 481513 | Selenic acid solution 40 wt. % in H2O, 99.95% trace metals basis | 7783-08-6 | 100ml | $287 | 2024-03-01 | Buy |
| Alfa Aesar | 018851 | Selenic acid, 40% aq. soln. | 7783-08-6 | 100g | $215 | 2024-03-01 | Buy |
| Alfa Aesar | 018851 | Selenic acid, 40% aq. soln. | 7783-08-6 | 500g | $948 | 2024-03-01 | Buy |
| Strem Chemicals | 93-3420 | Selenic acid, 40% solution | 7783-08-6 | 50g | $144 | 2024-03-01 | Buy |
Selenic acid Chemical Properties,Uses,Production
Chemical Properties
Anhydrous H2SeO4 forms colorless prismatic or needle-shaped crystals; extremely hygroscopic; the melt tends to supercool. On heating, decomposes into SeO2, O2 and H2O.
Uses
Selenic acid is used as an oxidizing agent. Its derivative sodium selenate is used in the preparation of glass and animal feeds. It acts as an analytical reagent and also used for the preparation of other selenium salts such as gold(III) selenate. Further, it reacts with fluorosulfuric acid to get selenoyl fluoride.
Definition
ChEBI: Selenic acid is a selenium oxoacid. It is a conjugate acid of a hydrogenselenate.
Preparation
SeO2 + H2O2 = H2SeO4
The oxidation is carried out in aqueous solution so that dilute
selenic acid solution is obtained first; this may be concentrated to
the anhydrous acid by evaporation.
General Description
A white crystalline solid. Very corrosive to skin, eyes and mucous membranes. Corrosive to metal. Toxic by skin absorption and by ingestion.
Air & Water Reactions
Very hygroscopic. Soluble in water.
Reactivity Profile
Selenic acid, LIQUID reacts exothermically with chemical bases (for example: amines and inorganic hydroxides) to form salts. The reactions can generate dangerously large amounts of heat in small spaces. A good oxidizing agent. May oxidize organic matter such as wood, cotton, fiberboard, etc.. Reacts with active metals, including iron and aluminum, and also less active metals, to dissolve the metal and liberate hydrogen and/or toxic gases. Can initiate polymerization in certain classes of organic compounds. Reacts with cyanide salts and compounds to release gaseous hydrogen cyanide. Flammable and/or toxic gases are also often generated with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and weak or strong reducing agents. Additional gas-generating reactions occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and even carbonates: the carbon dioxide gas from the last is nontoxic but the heat and spattering from the reaction can be troublesome. May often catalyze (increase the rate) of chemical reactions.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
Selenium compounds are poisons. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of Se. See also SELENIUM COMPOUNDS,
Selenic acid Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21628 | 55 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12806 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | laboratory@coreychem.com | China | 30230 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34563 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 |
| Aladdin Scientific | tp@aladdinsci.com | United States | 57505 | 58 | |
| XIAMEN AMITY INDUSTRY AND TRADE CO., LTD. | +8618950047208 | ellena@amitychem.com | China | 43416 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39927 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32343 | 55 |
View Lastest Price from Selenic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-03-21 | Selenic acid
7783-08-6
|
US $100.00 / kg | 1kg | 99% | 50000KG/month | Hebei Mujin Biotechnology Co.,Ltd |
-

- Selenic acid
7783-08-6
- US $100.00 / kg
- 99%
- Hebei Mujin Biotechnology Co.,Ltd