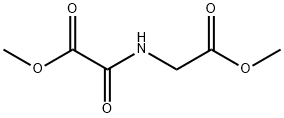
DMOG
- CAS No.
- 89464-63-1
- Chemical Name:
- DMOG
- Synonyms
- DIMETHYLOXALYLGLYCINE;OMOG;Dimethyloxaloylglycine;OMO;DMOG;DMOG, >=98%;DMOG USP/EP/BP;DIMETHYLOXALLYL GLYCINE;Dimethyloxalylglycine (Dmog);DiMethyloxaloylglycine (DMOG)
- CBNumber:
- CB8223849
- Molecular Formula:
- C6H9NO5
- Molecular Weight:
- 175.14
- MDL Number:
- MFCD05865098
- MOL File:
- 89464-63-1.mol
- MSDS File:
- SDS
| Melting point | 46-48°C |
|---|---|
| Boiling point | 179-182 °C(Press: 12 Torr) |
| Density | 1.246±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | H2O: >30mg/mL |
| pka | 10.55±0.46(Predicted) |
| form | Solid |
| color | white to off-white |
| Water Solubility | Soluble in water at 30mg/ml. |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions are not stable. Solutions must be made fresh and used within 1 working day. |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P301+P312+P330 |
| Hazard Codes | Xi,Xn |
| Risk Statements | 22 |
| WGK Germany | 3 |
| HS Code | 2924.19.8000 |
| HazardClass | IRRITANT |
DMOG price More Price(53)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | D3695 | DMOG ≥98% (HPLC) | 89464-63-1 | 10mg | $100 | 2024-03-01 | Buy |
| Sigma-Aldrich | 400091 | HIF-Hydroxylase Inhibitor, DMOG | 89464-63-1 | 50mg | $99.4 | 2024-03-01 | Buy |
| TCI Chemical | D5480 | Dimethyloxaloylglycine | 89464-63-1 | 50MG | $50 | 2024-03-01 | Buy |
| TCI Chemical | D5480 | Dimethyloxaloylglycine | 89464-63-1 | 250MG | $171 | 2024-03-01 | Buy |
| Alfa Aesar | J67077 | Dimethyloxaloylglycine, 98% | 89464-63-1 | 10mg | $32.65 | 2024-03-01 | Buy |
DMOG Chemical Properties,Uses,Production
Description
The pro-
Chemical Properties
Off-White to Pale Pink Solid
Uses
DMOG is a cell permeable prolyl-4-hydroxylase inhibitor that regulates hypoxia-inducible factor
Uses
DMOG is a cell permeable prolyl-4-hydroxylase inhibitor which upregulates HIF activity. HIF activation stimulates angiogenesis in several different models (1nM). DMOG also inhibits FIH (factor inhibiting HIF), an asparaginyl hydroxylase, which enhances the HIF response. It is active in vivo and attenuates myocardial injury in a rabbit ischemia reperfusion model (20mg/Kg).
Definition
ChEBI: Dimethyloxalylglycine is a glycine derivative that is the diester obtained by formal condensation of the carboxy groups of N-oxalylglycine with two molecules of methanol. It has a role as a neuroprotective agent and an EC 1.14.11.29 (hypoxia-inducible factor-proline dioxygenase) inhibitor. It is a glycine derivative, a methyl ester and a secondary carboxamide. It is functionally related to a N-oxalylglycine.
Biochem/physiol Actions
DMOG is a cell permeable prolyl-4-hydroxylase inhibitor, which upregulates HIF (hypoxia-inducible factor). The protein level of HIF-1α subunit is post-transcriptionally regulated by prolyl and asparaginyl hydroxylase (PAH). Suppression of PAH activity increases endogenous HIF-1α levels. DMOG is a cell permeable, competitive inhibitor of prolyl hydroxylase domain-containing proteins (PHDs and HIF-PHs). It has been discovered that the DMOG posseses neuroprotective effect on NFG deprived cell cultures through preservation of glucose metabolism. DMOG also attenuates myocardial injury in a rabbit ischemia reperfusion model. DMOG is more potent than the older inhibitor 4-Phenyl-pyridine-2,5-dicarboxylic acid (R395889; Sigma-Aldrich rare chemicals library). The IC50 is 5.18 μM.
in vitro
dmog acts to stabilize hif-1a expression under normal oxygen tension in cultured cells at concentrations from 0.1 to 1 mmol/l [2].
in vivo
pre-treatment with dmog attenuates systemic lps-induced activation of the nf-κb pathway. furthermore, mice treated with dmog had significantly increased survival in lps-induced shock. in addition, in vivo dmog treatment upregulates the expression of il-10, specifically in the peritoneal b-1 cell population [3].
Enzyme inhibitor
This iron-binding a-ketoglutarate (2-oxoglutarate) analogue (FW = 147.09 g/mol; also named N-oxaloglycine) competitively inhibits prolyl-4- hydroxylase (Reaction: Procollagen (L-proline) + a-ketoglutarate + O2 → Procollagen ( (2S,4R) -4-hydroxyproline) + succinate + CO2). During catalysis, prolyl-4-hydroxylase forms Fe (III), and the latter most likely makes an extremely stable metal ion complex with N-oxaloglycine. Substitution on the glycine moiety alters inhibitor activity stereoselectively and that, if the w-carboxylate is homologated or replaced, either by acylsulfonamides or anilide, activity is likewise sharply reduced. Prolyl- 4-Hydroxylase Catalysis: Each catalytic round of this posttranslational modifying enzyme reaction occurs in two stages. O2 is bound end-on in an axial position, producing a dioxygen unit. Nucleophilic attack at C-2 generates a tetrahedral intermediate, with loss of the double bond in dioxygen and bonds to iron and the a-carbon of a-ketoglutarate. Elimination of CO2 coincides with formation of the Fe (IV) =O species. The second stage involves the abstraction of the pro-R hydrogen atom from C-4 of proline, followed by radical combination, yielding hydroxyproline. In the presence of a-ketoglutarate, enzyme-bound Fe2+ is rapidly converted to Fe3+, resulting in inactivation of the enzyme Ascorbate is utilized as a cofactor to reduce Fe (III) back to Fe (II). Cell-Permeable Analogue: Dimethyloxalylglycine (FW = 175.14 g/mol; CAS 89464-63-1; Symbol: DMOG, also named N- (methoxyoxoacetyl) -glycine methyl ester) is metabolicaly demethylated to form N-oxaloglycine upon entry to many cells.
IC 50
9.3 and 3.7 μm for hydroxyproline synthesis inhibition of embryonic chicken lung extracted from tissue and culture medium [1].
storage
Store at -20°C
References
1) Asikainen et al. (2005), Activation of hypoxia-inducible factors in hyperoxia through prolyl 4-hydroxylase blockade in cells and explants of primate lung; Proc. Natl. Acad. Sci. USA, 102 10212 2) Jaakkola et al. (2001), Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation; Science, 292 468 3) Hamada et al. (2009), Synthesis and activity of N-oxalylglycine and it’s derivatives as jumonji C-domain-containing histone lysine demethylase inhibitors; Bioorg. Med. Chem. Lett., 19 2852 4) Lomb et al. (2009), Prolyl hydroxylase inhibitors depend on extracellular glucose and hypoxia-inducible factor (HIF)-2alpha to inhibit cell death caused by nerve growth factor deprivation: evidence that HIF-2alpha has a role in NGF-promoted survival of sympathetic neurons; Mol. Pharmacol., 75 1198 5) Xie et al. (2012), PHD3-dependent hydroxylation of HCLK2 promotes the DNA damage response; J. Clin. Invest., 122 2827
DMOG Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29881 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| SHANGHAI T&W PHARMACEUTICAL CO., LTD. | +86-021-61551413 +8618813727289 | contact@trustwe.com | China | 5738 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32165 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 22783 | 58 |
| Nextpeptide Inc | +86-0571-81612335 +8613336028439 | sales@nextpeptide.com | China | 19908 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6391 | 58 |
| Coresyn Pharmatech Co., Ltd. | +86-571-86626709 +86-18157142896 | cbc@coresyn.com | China | 9984 | 58 |
| ShenZhen Trendseen Biological Technology Co.,Ltd. | 13417589054 | trendseenbio@gmail.com | China | 11681 | 58 |