PD173955
- CAS No.
- 260415-63-2
- Chemical Name:
- PD173955
- Synonyms
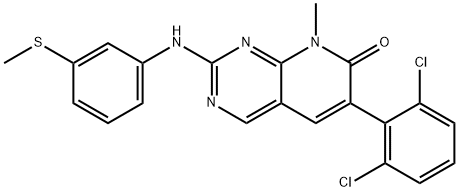
- CS-1508;PD-173955;CHEBI:49791;PD173955 USP/EP/BP;PD173955, 10 mM in DMSO;PD173955; CHEBI:49791; 260415-63-2; PD-173955;6-(2,6-dichlorophenyl)-8-methyl-2-(3-methylsulfanylanilino)pyrido[2,3-d]pyrimidin-7-one;6-(2,6-Dichlorophenyl)-8-methyl-2-((3-(methylthio)phenyl)amino)pyrido[2,3-d]pyrimidin-7(8H)-on;6-(2,6-Dichlorophenyl)-8-methyl-2-[[3-(methylthio)phenyl]amino]pyrido[2,3-d]pyrimidin-7(8H)-one;Pyrido[2,3-d]pyrimidin-7(8H)-one, 6-(2,6-dichlorophenyl)-8-methyl-2-[[3-(methylthio)phenyl]amino]-
- CBNumber:
- CB82485076
- Molecular Formula:
- C21H16Cl2N4OS
- Molecular Weight:
- 443.35
- MDL Number:
- MFCD16038305
- MOL File:
- 260415-63-2.mol
- MSDS File:
- SDS
PD173955 price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| ApexBio Technology | A8812 | PD173955 | 260415-63-2 | 1unit | $28 | 2021-12-16 | Buy |
| ApexBio Technology | A8812 | PD173955 | 260415-63-2 | 5mg | $138 | 2021-12-16 | Buy |
| Adipogen Life Sciences | SYN-1061-M005 | PD-173955 ≥95% | 260415-63-2 | 5mg | $297 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | BRS0000356 | PD-173955 95.00% | 260415-63-2 | 1MG | $308.7 | 2021-12-16 | Buy |
| ChemScene | CS-3550 | PD173955 98.88% | 260415-63-2 | 10mg | $336 | 2021-12-16 | Buy |
PD173955 Chemical Properties,Uses,Production
Uses
PD173955 is an orally active inhibitor of Src (IC50= 22 nM), Yes, Abl, ATP and MAP kinases. PD173955 can effectively prevent the mitotic process and has anticancer activity[1][2][3][4].
Definition
ChEBI: PD173955 is a dichlorobenzene, a methyl sulfide, a pyridopyrimidine and an aryl sulfide. It has a role as a tyrosine kinase inhibitor.
Biological Activity
pd173955 is a potent inhibitor of bcr-abl, src and yes with ic50 value of 1-2 nm, 300 nm and 175 nm, respectively [1-3].bcr-abl is a protein tyrosine kinase which has oncogenic potential. src is an enzyme and plays an important role in a variety of cancer cells survival, angiogenesis, proliferation and invasion pathways. yes is a proto-oncogene tyrosine-protein kinase smf belongs to the src kinase family [1-3].pd173955 is a potent bcr-abl, src and yes inhibitor. when tested with cml cd34+ gm progenitors, pd173955 inhibited kl-dependent proliferation at an ic50 value of 50 nm and gm-csf-dependent cell growth at an ic50 value of 1μm and the maximum inhibition was achieved at the dose of 25 nm. it was shown that pd173955 reduced the fractions of cells in g2-m phase and increased the cells in g1 phase with significant difference [2]. in ht29 cells, pd173955 treatment inhibited src auot-phosphorylation in a dose dependent manner. further, pd173955 showed inhibition on cell growth with ic50 value of 800 nm without morphologic changes and high concentrations arrested cell cycle at the m phase [1]. when tested with bcr-abl-depedent cell lines k562 and rwleu4, pd173955 showed inhibition on cell proliferation with the ic50 value of 35 and 10 nm, respectively and arrested cell cycle in g1 phase at the low nanomolar [3].
Enzyme inhibitor
This tyrosine kinase inhibitor (FW = 443.35 g/mol; CAS 260415-63-2; Solubility: <1 mg/mL DMSO, Ethanol, H2O), 6-(2,6-dichlorophenyl)-8- methyl-2-((3-(methylthio)phenyl)amino)pyrido[2,3-d]pyrimidin-7(8H)-one targets Bcr-Abl (IC50 = 1-2 nM, in vitro), the constitutively active tyrosine kinase that results from inadvertent fusion of bcr and abl genes and that causes oncogenic transformation in chronic myelogenous leukemia, or CML, inhibiting Bcr-Abl-dependent cell growth with an IC50 of 2-35 nM in different cell lines. PD173955 also inhibits kit ligand-dependent c-kit autophosphorylation (IC50 = ~25 nM) and kit ligand-dependent proliferation of M07e cells (IC50 = 40 nM), but had a lesser effect on interleukin 3- dependent (IC50 = 250 nM) or granulocyte macrophage colony-stimulating factor (IC50 = 1 μM)-dependent cell growth. PD173955 also increases the susceptibility of HT29 cells to detachment-induced apoptosis (anoikis) in a dose- and time-dependent manner. Structural Features Distinguishing Imatinib & PD173955 Binding: Crystal structures of Abl kinase domain complexes with imatinib (Gleevec) and PD173955 show that both bind to the canonical ATP-binding site, but in distinctive ways. Imatinib captures a specific inactive conformation of Abl’s activation loop, mimicking the bound peptide substrate. In contrast, PD173955 binds to the Abl activation loop in a way that resembles the active kinase conformation. The 10x greater potency of PD173955 over imatinib is attributed to its ability to target multiple active and inactive forms of Abl, whereas imatinib binds only to a specific catalytically inactive conformation (4
in vivo
PD166326 (50 mg/kg; Oral gavage; Twice daily) has anti-leukemia activity in CML mice[4].
| Animal Model: | Balb/c mice model of chronic myeloid leukemia (CML)[4] |
| Dosage: | 50 mg/kg |
| Administration: | Oral gavage (p.o.), Twice daily for 4 days |
| Result: | Significantly inhibited constitutive tyrosine phosphorylation of numerous proteins (including Lyn) in primary Bcr/abl expressing leukemia cells. Reduced the number of mice with splenomegaly and inhibited splenomegaly in at least half of the mice compared to the control group. Was effective in reducing the peripheral blood granulocytosis of the murine CML-like disease. |
References
[1]. windham, t.c., et al., src activation regulates anoikis in human colon tumor cell lines. oncogene, 2002. 21(51): p. 7797-807.
[2]. strife, a., et al., direct evidence that bcr-abl tyrosine kinase activity disrupts normal synergistic interactions between kit ligand and cytokines in primary primitive progenitor cells. mol cancer res, 2003. 1(3): p. 176-85.
[3]. wisniewski, d., et al., characterization of potent inhibitors of the bcr-abl and the c-kit receptor tyrosine kinases. cancer res, 2002. 62(15): p. 4244-55.
PD173955 Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29730 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19552 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49732 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32202 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 22854 | 58 |
| Hefei Hirisun Pharmatech Co., Ltd | +8615056975894 | shawn@hirisunpharm.com | CHINA | 9911 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6391 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24727 | 58 |