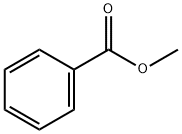
Methyl benzoate
- CAS No.
- 93-58-3
- Chemical Name:
- Methyl benzoate
- Synonyms
- BENZOIC ACID METHYL ESTER;NIOBE OIL;FEMA 2683;Oilofmiobe;Oniobe oil;Oxidate le;OIL OF NIOBE;Methylbenzoat;Slethylbenzoat;METHYL BENZOATE
- CBNumber:
- CB8252119
- Molecular Formula:
- C8H8O2
- Molecular Weight:
- 136.15
- MDL Number:
- MFCD00008421
- MOL File:
- 93-58-3.mol
- MSDS File:
- SDS
| Melting point | -12 °C (lit.) |
|---|---|
| Boiling point | 198-199 °C (lit.) |
| Density | 1.088 g/mL at 20 °C (lit.) |
| vapor density | 4.68 (vs air) |
| vapor pressure | <1 mm Hg ( 20 °C) |
| refractive index |
n |
| FEMA | 2683 | METHYL BENZOATE |
| Flash point | 181 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | ethanol: soluble60%, clear (1mL/4ml) |
| form | Liquid |
| color | Clear colorless to pale yellow |
| Specific Gravity | 1.087~1.095 (20℃) |
| Odor | at 1.00 % in dipropylene glycol. phenolic wintergreen almond floral cananga |
| Odor Type | phenolic |
| explosive limit | 8.6-20%(V) |
| Water Solubility | <0.1 g/100 mL at 22.5 ºC |
| Merck | 14,6024 |
| JECFA Number | 851 |
| BRN | 1072099 |
| Dielectric constant | 6.6(20℃) |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, strong acids, strong bases. |
| LogP | 2.12-2.2 at 20℃ |
| Substances Added to Food (formerly EAFUS) | METHYL BENZOATE |
| FDA 21 CFR | 172.515 |
| CAS DataBase Reference | 93-58-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 6618K1VJ9T |
| NIST Chemistry Reference | Benzoic acid, methyl ester(93-58-3) |
| EPA Substance Registry System | Methyl benzoate (93-58-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P501 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22 | |||||||||
| Safety Statements | 36 | |||||||||
| RIDADR | UN 2938 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | DH3850000 | |||||||||
| Autoignition Temperature | 510 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29163100 | |||||||||
| Hazardous Substances Data | 93-58-3(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in rats: 3.43 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Methyl benzoate price More Price(46)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W268313 | Methyl benzoate natural, ≥98%, FCC, FG | 93-58-3 | 100g | $146 | 2024-03-01 | Buy |
| Sigma-Aldrich | W268313 | Methyl benzoate natural, ≥98%, FCC, FG | 93-58-3 | 1kg | $964 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHR2475 | Guaiacol Impurity E Pharmaceutical Secondary Standard; Certified Reference Material | 93-58-3 | 100MG | $534 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.22330 | Methyl benzoate for synthesis | 93-58-3 | 100mL | $18.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.22330 | Methyl benzoate for synthesis | 93-58-3 | 1L | $90.2 | 2024-03-01 | Buy |
Methyl benzoate Chemical Properties,Uses,Production
Description
Methyl benzoate is an organic compound. It is an ester with the chemical formula C6H5CO2CH3. It is a colorless liquid that is poorly soluble in water, but miscible with organic solvents. Methyl benzoate has a pleasant smell, strongly reminiscent of the fruit of the feijoa tree, and it is used in perfumery. It also finds use as a solvent and as a pesticide used to attract insects such as orchid bees.
Chemical Properties
Methyl benzoate is a colorless, oily, transparent liquid with a pleasant, fruity odor similar to ylang-ylang. It is soluble in methanol and ether, insoluble in water and glycerin, and miscible with ether. It is used in perfume bases such as ylang-ylang and tuberose types.
Occurrence
Methyl benzoate can be isolated from the freshwater fern Salvinia molesta. It is one of many compounds that is attractive to males of various species of orchid bees, which apparently gather the chemical to synthesize pheromones; it is commonly used as bait to attract and collect these bees for study.
Cocaine hydrochloride hydrolyzes in moist air to give methyl benzoate; drug - sniffing dogs are thus trained to detect the smell of methyl benzoate.
Uses
Methyl benzoate is used in perfumery. It also finds use as a solvent. It acts as an intermediate and odor agents. Further, it is used to attract insects such as orchid bees. It is also used for cellulose esters, cellulose ethers, synthetic resin and rubber solvent and polyester fibers to assist in the preparation of flavor.
Definition
ChEBI: Methyl benzoate is a benzoate ester obtained by condensation of benzoic acid and methanol. It has a role as a metabolite and an insect attractant. It is a benzoate ester and a methyl ester.
Preparation
Methyl benzoate is manufactured by heating benzoic acid and dimethyl sulfate to high temperature, or by exchange between ethyl benzoate and methanol in KOH solution. It may also be produced by the alcoholysis of benzonitrile. It is a by-product of ozonolysis of water.
Application
Methyl benzoate is a volatile aromatic ester compound widely used in perfumery industries. It is naturally occurring in guava, mango, and kiwifruit. It is one of many compounds that is attractive to males of various species of orchid bees, who apparently gather the chemical to synthesize pheromones. It can also be utilized as a precursor for:
Selective synthesis of benzaldehyde using supported manganese oxide catalysts.
Preparation of benzophenone derivatives by reacting with aryl compounds via Friedel-Crafts acylation.
Aroma threshold values
Detection: 110 ppb
Taste threshold values
Taste characteristics at 30 ppm: phenolic and cherry pit with a camphoraceous nuance.
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 31, p. 4209, 1983 DOI: 10.1248/cpb.31.4209
General Description
A crystalline solid or a solid dissolved in a liquid. Denser than water. Contact may slightly irritate skin, eyes and mucous membranes. May be slightly toxic by ingestion. Used to make other chemicals.
Air & Water Reactions
Slightly soluble in water. Hydrolyzes slowly in contact with water .
Reactivity Profile
Methyl benzoate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Methyl benzoate reacts with strong oxidizing agents and strong bases and hydrolyzes slowly in contact with water. .
Health Hazard
Methyl benzoate is a mild skin irritant. Irritating to the eyes, nose, throat, upper respiratory tract, and skin. May cause allegic skin and respiratory reactions.Theacute oral toxicity in test animals was oflow order. The toxic symptoms in animalsfrom oral administration of this compoundwere tremor, excitement, and somnolence.The LD50 value varies with species. The oralLD50 values in mice and rats are 3330 and1350 mg/kg, respectively.
Safety Profile
Moderately toxic by ingestion. Mildly toxic by skin contact. A skin and eye irritant. Combustible liquid when exposed to heat or flame; can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical, water to blanket fire. When heated to decomposition it emits acrid smoke and irritating fumes.
Synthesis
Methyl benzoate is formed by the condensation of methanol and benzoic acid, in presence of a strong acid such as hydrochloric acid . It reacts both at the ring and the ester. Illustrative of its ability to undergo electrophilic substitution, methyl benzoate undergoes acidcatalysed nitration with nitric acid to give methyl 3-nitrobenzoate. It also undergoes hydrolysis with addition of aqueous NaOH to give methanol and sodium benzoate, which can be acidified with aqueous HCl to form benzoic acid.
Potential Exposure
Used as food additive and as a solvent for cellulose esters and ethers, resins and rubber.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. Note to physician: Inhalation: Bronchodialators, decongestants, and oxygen may be used if necessary. Corticosteroids are useful for treating pneumonitis.
storage
Color Code—Red: Flammability Hazard: Store in a flammable liquid storage area or approved cabinet away from ignition sources and corrosive and reactive materials. Prior to working with methyl benzoate you should be trained on its proper handling and storage. Store in tightly closed containers in a cool, well-ventilated area away from strong Methyl benzoate 1761 acids, strong bases, oxidizers, nitrates. Where possible, automatically pump liquid from drums or other storage containers to process containers. Drums must be equipped with selfclosing valves, pressure vacuum bungs, and flame arresters. Use only nonsparking tools and equipment, especially when opening and closing containers of this chemical. Wherever this chemical is used, handled, manufactured, or stored, use explosion-proof electrical equipment and fittings.
Shipping
Methyl benzoate requires a shipping label of “POISONOUS/TOXIC MATERIALS.” It falls in Hazard Class 6.1 and Packing Group III
Purification Methods
Wash the ester with dilute aqueous NaHCO3, then water, dry with Na2SO4 and fractionally distil it in a vacuum. [Beilstein 9 IV 283.]
Incompatibilities
Incompatible with strong acids, strong bases, nitrates, oxidizers.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Methyl benzoate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuhan Biet Co., Ltd. | +8617320528784 | min@biet.com.cn | China | 41 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5889 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12837 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| China Synchem Technology Co.,Ltd. | +86-0552-4929304 +86-18055277008 | wangxuhui1985@126.com | China | 57 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1001 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29880 | 58 |
Related articles
- What is the nitration process of Methyl benzoate?
- Methyl m-nitrobenzoate (methyl 3-nitrobenzoate) is an organic synthesis intermediate. It can be used as a starting material fo....
- Dec 16,2024
- The brief introduction of Methyl benzoate
- Methyl benzoate is a volatile ester that exists naturally as a metabolite in plants.
- Jun 21,2024
View Lastest Price from Methyl benzoate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Methyl benzoate
93-58-3
|
US $10.00 / kg | 1kg | 99% | 100 mt | Hebei Weibang Biotechnology Co., Ltd | |
 |
2024-10-25 | methyl benzoate
93-58-3
|
US $0.00 / KG | 1KG | 0.99 | 1000kg | Hebei Yanxi Chemical Co., Ltd. | |
 |
2024-10-11 | Methyl benzoate
93-58-3
|
US $10.00 / KG | 1KG | 99.% | 10 ton | Hebei Chuanghai Biotechnology Co,.LTD |
-

- Methyl benzoate
93-58-3
- US $10.00 / kg
- 99%
- Hebei Weibang Biotechnology Co., Ltd
-

- methyl benzoate
93-58-3
- US $0.00 / KG
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- Methyl benzoate
93-58-3
- US $10.00 / KG
- 99.%
- Hebei Chuanghai Biotechnology Co,.LTD