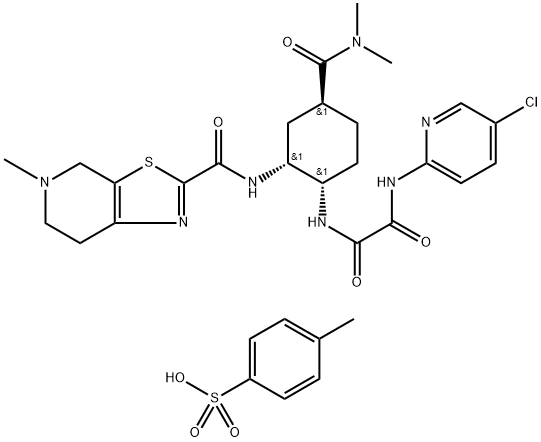
Edoxaban tosylate monohydrate
- CAS No.
- 1229194-11-9
- Chemical Name:
- Edoxaban tosylate monohydrate
- Synonyms
- Edoxaban tosilate;edoxaban tosylate hydrate;Edoxaban Tosilate Monohydrate;Lixiana;Ethanediamide, N1-(5-chloro-2-pyridinyl)-N2-[(1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-[[(4,5,6,7-tetrahydro-5-methylthiazolo[5,4-c]pyridin-2-yl)carbonyl]amino]cyclohexyl]-, 4-methylbenzenesulfonate, hydrate;CS-1597;Yidu Saban Mesylate;Edoxaban Tosylate Hydrate 1;Etuxaban p-toluenesulfonate;Edoxaban (TsOH salt hydrate)
- CBNumber:
- CB82645582
- Molecular Formula:
- C31H38ClN7O7S2
- Molecular Weight:
- 720.26
- MDL Number:
- MFCD28400751
- MOL File:
- 1229194-11-9.mol
- MSDS File:
- SDS
| Melting point | >252°C (dec.) |
|---|---|
| storage temp. | Hygroscopic, Refrigerator, under inert atmosphere |
| solubility | Chloroform (Very Slightly), DMSO (Slightly), Methanol (Slightly, Sonicated) |
| form | Solid |
| color | White to Off-White |
| Stability | Hygroscopic |
| InChIKey | ZLFZITWZOYXXAW-BUZWFPPCNA-N |
| SMILES | S(C1C=CC(C)=CC=1)(O)(=O)=O.N([C@@H]1C[C@@H](C(=O)N(C)C)CC[C@@H]1NC(=O)C(=O)NC1N=CC(Cl)=CC=1)C(C1SC2CN(C)CCC=2N=1)=O |&1:12,14,22,r| |
| FDA UNII | 972203R4EW |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
Edoxaban tosylate monohydrate price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | E555525 | EdoxabanTosylateHydrate | 1229194-11-9 | 100mg | $195 | 2021-12-16 | Buy |
| TRC | E555525 | EdoxabanTosylateHydrate | 1229194-11-9 | 1g | $690 | 2021-12-16 | Buy |
| Usbiological | 448075 | Edoxaban Tosylate Monohydrate | 1229194-11-9 | 50mg | $418 | 2021-12-16 | Buy |
| ApexBio Technology | A3383 | Edoxabantosylatemonohydrate | 1229194-11-9 | 200mg | $680 | 2021-12-16 | Buy |
| ChemScene | CS-1333 | Edoxabantosylatemonohydrate 99.95% | 1229194-11-9 | 50mg | $220 | 2021-12-16 | Buy |
Edoxaban tosylate monohydrate Chemical Properties,Uses,Production
Synthesis Method
The convergent synthesis of
edoxiban tosilate (XI) involves the union of three key structural subunits, diamino cyclohexane intermediate 127, pyridyl amino
oxoacetate 128, and thiazole acid 129 (Scheme 1) and the discovery
synthesis and related compounds were disclosed in several
publications. Several patents on the improved synthesis of
the diamino and thiazole intermediates have been disclosed, including an improved synthesis of edoxaban. Because
the synthesis described in the latest patents do not involve
any chromatographic purification, this is the most likely processscale
route, and will be highlighted (Scheme 1–3).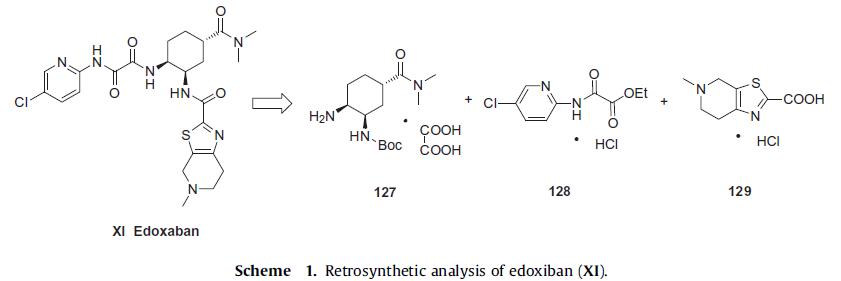
The preparation of the tetrahydropyridyl thiazolo acid 129 is
shown in Scheme 2. N-Methyl piperidone (130) is treated with
catalytic pyrrolidine, cyanamide, and sulfur in warm isopropanol to yield aminothiazole 131. Diazotization of thiazole amine 131
in the presence of 48% HBr with sodium nitrite at 30°C gave the
thiazole bromide, which was directly converted to tosylate salt
132 in 78% yield from piperidone 130. After free-basing the salt
with sodium hydroxide, the resulting bromide was treated with
n-BuLi followed by bubbling carbon dioxide gas to give lithium carboxylate
salt 133. Acidification of this salt in ethanolic HCl gave the
desired thiazole acid hydrochloride salt (129) in 90% yield over two
steps.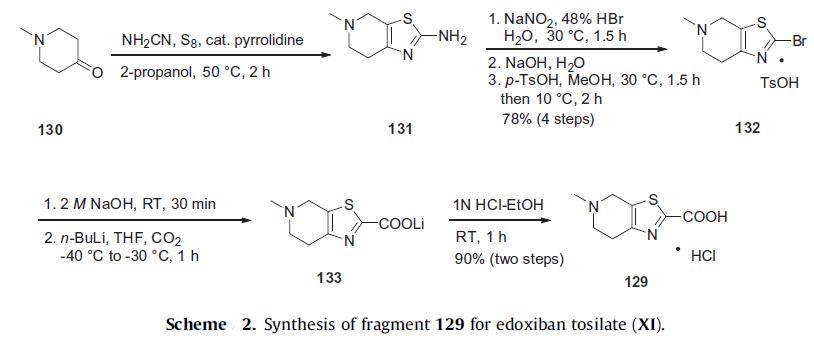
The synthesis of edoxaban tosilate is shown in Scheme 3. Commercially
available epoxide ester 134 was reacted with sodium
azide to afford the corresponding hydroxyazide intermediate regiospecifically and in quantitative yield. This intermediate was
immediately subjected to catalytic hydrogenation in the presence
of Boc2O to provide alcohol 135 in 68% yield. Alcohol 135 was converted
to the corresponding mesylate, followed by the treatment
with sodium azide to give primarily cis-azide 136 in 32% yield over
two steps after separation of diastereomers. Reduction of the azide
followed by a Cbz protection provided carbamate 137 in 67% yield
over two steps. The ester group of 137 was hydrolyzed with lithium
hydroxide and reacted with dimethylamine to furnish N,N-dimethylamide
138 in 76% yield over the two step sequence. The Cbz group
of 138 was then removed by catalytic hydrogenation and the resulting
amine was immediately converted to oxalate salt 127.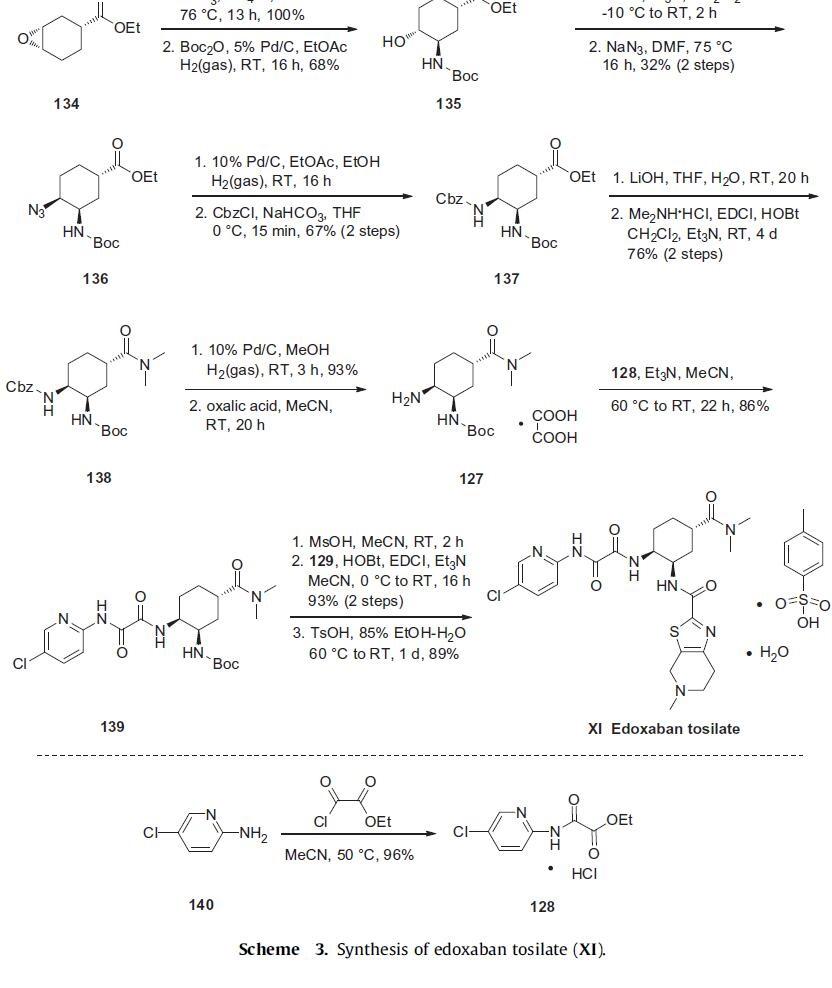
Amine 127 was then condensed with the key pyridylamino oxoacetate
128, prepared in 96% yield by reacting 5-chloropyridin-2-amine
(140) with ethyl 2-chloro-2-oxoacetate in warm acetonitrile, to provide
amide 139 in 86% yield. Finally, removal of the Boc group of 139
with methanesulfonic acid and EDCI-mediated coupling with tetrahydropyridyl thiazolo acid 129 gave edoxaban in 93% yield. Treatment
with TsOH led to isolation of edoxaban tosilate (XI).
Description
Edoxaban tosylate monohydrate is an orally active, highly potent, selective, and direct Factor Xa (FXa) inhibitor with Kis of 0.561 and 2.98 nM for free human FXa and prothrombinase. Edoxaban monohydrate exhibits more than 10,000-fold selectivity over other coagulation proteases. Edoxaban monohydrate can be used for preventing thromboembolic disease research.
Uses
Edoxaban is an anticoagulant drug which acts as a direct factor Xa inhibitor.
Definition
ChEBI: A hydrate that is the monohydrate of the tosylate salt of edoxaban. Used for the treatment of deep vein thrombosis and pulmonary embolism.
Biological Activity
factor xa (fxa), a key serine protease, is a promising target enzyme for the prophylaxis and treatment of thromboembolic diseases. edoxaban tosylate monohydrate is a novel antithrombotic agent that directly inhibits fxa activity.
Clinical Use
Daichi Sankyo’s edoxaban tosilate is an orally administered coagulation factor Xa inhibitor that was approved and launched in Japan for the preventive treatment of venous thromboembolic events (VTE) in patients undergoing total knee arthroplasty, total hip arthroplasty, or hip fracture surgery. Edoxaban has been shown to have a rapid onset of anticoagulant effect due to short Tmax (1–2 h) after dosing and sustained for up to 24 h post-dose. Marketed under the brand name Lixiana, it is currently in phase III studies in the US for the prevention of stroke and systemic embolic events in patients with atrial fibrillation (AF) and venous thromboembolism (VTE).
in vitro
edoxaban tosylate monohydrate (du-176b) inhibited fxa with ki values of 0.561 nm for free fxa, 2.98 nm for prothrombinase, and exhibited >10 000-fold selectivity for fxa. du-176b doubled prothrombin time and activated partial thromboplastin time in human plasma. du-176b did not impair platelet aggregation by adp, collagen or u46619 [1].
in vivo
du-176b dose-dependently inhibited thrombus formation in rat and rabbit thrombosis models, although bleeding time in rats was not significantly prolonged at an antithrombotic dose [1].
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: increased risk of bleeding with NSAIDs
and high dose aspirin; increased risk of haemorrhage
with IV diclofenac and ketorolac - avoid
Anti-arrhythmics: concentration increased by
dronedarone (reduce edoxaban dose)
Antibacterials: concentration increased by
erythromycin (reduce edoxaban dose); concentration
reduced by rifampicin.
Anticoagulants: increased risk of haemorrhage with
other anticoagulants - avoid.
Antidepressants: concentration possibly reduced by
St John’s wort.
Antiepileptics: concentration possibly reduced
by carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone.
Antifungals: concentration increased by ketoconazole
(reduce edoxaban dose).
Ciclosporin: concentration of edoxaban increased
(reduce edoxaban dose).
Metabolism
Unchanged edoxaban is main form in plasma.
Edoxaban is metabolised via hydrolysis (mediated
by carboxylesterase 1), conjugation or oxidation by
CYP3A4/5 (<10%). Edoxaban has 3 active metabolites,
the predominant metabolite (M-4), formed by hydrolysis,
is active and reaches less than 10% of the exposure of the
parent compound in healthy subjects. Exposure to the
other metabolites is less than 5%. Edoxaban is a substrate
for the efflux transporter P-glycoprotein (P-gp), but
not a substrate for uptake transporters such as organic
anion transporter polypeptide OATP1B1, organic
anion transporters OAT1 or OAT3 or organic cation
transporter OCT2. Its active metabolite is a substrate for
OATP1B1.
Renal clearance accounts for approximately 35% of the
administered dose. Metabolism and biliary/intestinal
excretion account for the remaining clearance.
References
[1] furugohri t, isobe k, honda y, kamisato-matsumoto c, sugiyama n, nagahara t, morishima y, shibano t. du-176b, a potent and orally active factor xa inhibitor: in vitro and in vivo pharmacological profiles. j thromb haemost. 2008;6(9):1542-9.
[2] bathala ms, masumoto h, oguma t, he l, lowrie c, mendell j. pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor xa inhibitor, in humans. drug metab dispos. 2012;40(12):2250-5.
Edoxaban tosylate monohydrate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Zhuhai Hairuide Bioscience and Technology Co., Ltd | +8618928027945 | sales3@zhhairuide.com | China | 97 | 58 |
| Jinan Million Pharmaceutical Co., Ltd | 0531-68659554 +8613031714605 | info@millionpharm.com | China | 294 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 | sales@sjar-tech.com | China | 485 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Zibo Wei Bin Import & Export Trade Co. Ltd. | +86-0533-2091136 +8613864437655 | ziboweibinmaoyi@163.com | China | 100 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 973 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29730 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-+86-371-66670886 | info@dakenam.com | China | 19819 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1803 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21631 | 55 |
View Lastest Price from Edoxaban tosylate monohydrate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-03-31 | Edoxaban (tosylate Monohydrate)
1229194-11-9
|
US $2.00 / kg | 1kg | 99% | 200KG/M | Jinan Million Pharmaceutical Co., Ltd | |
 |
2025-03-29 | Edoxaban tosylate Monohydrate
1229194-11-9
|
US $0.00-0.00 / Kg/Drum | 1KG | 99%min | 1000kg | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2025-01-13 | Edoxaban Tosylate Monohydrate
1229194-11-9
|
US $0.00 / g | 1g | More Than 99% | 50kg/Month | BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. |
-

- Edoxaban (tosylate Monohydrate)
1229194-11-9
- US $2.00 / kg
- 99%
- Jinan Million Pharmaceutical Co., Ltd
-

- Edoxaban tosylate Monohydrate
1229194-11-9
- US $0.00-0.00 / Kg/Drum
- 99%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Edoxaban Tosylate Monohydrate
1229194-11-9
- US $0.00 / g
- More Than 99%
- BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
1229194-11-9(Edoxaban tosylate monohydrate)Related Search:
1of4