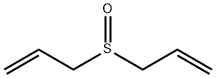
Diallyl sulfide
- CAS No.
- 592-88-1
- Chemical Name:
- Diallyl sulfide
- Synonyms
- ALLYL SULFIDE;Oil garlic;DIALLYL SULPHIDE;FEMA 2042;ALLYL SULPHIDE;Allyl monosulfide;di-2-propenyl sulfide;Diallyl sulfide, 97% / Allyl sulfide;NSC 20947;oilgarlic
- CBNumber:
- CB8356893
- Molecular Formula:
- C6H10S
- Molecular Weight:
- 114.21
- MDL Number:
- MFCD00008658
- MOL File:
- 592-88-1.mol
- MSDS File:
- SDS
| Melting point | -83 °C |
|---|---|
| Boiling point | 138 °C(lit.) |
| Density | 0.887 g/mL at 25 °C(lit.) |
| vapor density | 3.9 (vs air) |
| vapor pressure | 7 mm Hg ( 20 °C) |
| FEMA | 2042 | ALLYL SULFIDE |
| refractive index |
n |
| Flash point | 115 °F |
| storage temp. | 2-8°C |
| solubility | 3mg/mL in ethanol;5mg/mL in DMSO;10mg/mL in dimethyl formamide |
| form | Liquid |
| color | Clear colorless |
| Odor | at 0.10 % in propylene glycol. sulfurous onion garlic horseradish metallic |
| Odor Type | sulfurous |
| Odor Threshold | 0.00022ppm |
| explosive limit | 1.1%(V) |
| Water Solubility | Soluble in alcohol, chloroform, ether, and carbon tetrachloride. Insoluble in water. |
| Merck | 14,297 |
| JECFA Number | 458 |
| BRN | 1736016 |
| Dielectric constant | 4.9(20℃) |
| InChIKey | UBJVUCKUDDKUJF-UHFFFAOYSA-N |
| LogP | 2.61 |
| Substances Added to Food (formerly EAFUS) | ALLYL SULFIDE |
| FDA 21 CFR | 172.515 |
| CAS DataBase Reference | 592-88-1(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 60G7CF7CWZ |
| NIST Chemistry Reference | 1-Propene, 3,3'-thiobis-(592-88-1) |
| EPA Substance Registry System | 1-Propene, 3,3'-thiobis- (592-88-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS02 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H226 | |||||||||
| Precautionary statements | P210-P370+P378 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 10-36/37/38 | |||||||||
| Safety Statements | 26-36-37/39-23-16 | |||||||||
| RIDADR | UN 1993 3/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | BC4900000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29309070 | |||||||||
| NFPA 704 |
|
Diallyl sulfide price More Price(20)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W204218 | Allyl sulfide ≥97%, FG | 592-88-1 | 100g | $70.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | W204218 | Allyl sulfide ≥97%, FG | 592-88-1 | 1kg | $429 | 2024-03-01 | Buy |
| Sigma-Aldrich | W204218 | Allyl sulfide ≥97%, FG | 592-88-1 | 4kg | $1310 | 2024-03-01 | Buy |
| Sigma-Aldrich | 40647 | Allyl sulfide analytical standard | 592-88-1 | 1ml | $63.9 | 2022-05-15 | Buy |
| Sigma-Aldrich | 40647 | Allyl sulfide analytical standard | 592-88-1 | 5ml | $259 | 2022-05-15 | Buy |
Diallyl sulfide Chemical Properties,Uses,Production
General Description
Diallyl sulfide refers to the following 4 ingredients: diallyl monosulfide, diallyl disulfide, diallyl trisulfide and diallyl tetrasulfide, which exist in putrefaction decomposer of vegetables, green onion and the fruit of.cruciferous plants. As the main component of garlic extract, it is featured with strong anti-cancer, anti-virus, antibacterial activity, strong inhibition of platelet aggregation and immunity improvement. It has been widely used in the industries such as medical and health, fodder and the like.
Physical & Chemical Properties
It is an oily substance scented with garlic. Boiling point: 139 ° C (101.056Pa), relative density: 0.8876, refractive index: 1.4877. Soluble in organic solvents such as ether and the like, insoluble in water. Also it can be oxidized to diallyl sulfone.
Figure 1: Diallyl Disulfide
Figure 2: Diallyl trisulfide
Preparation Method
The two main methods for preparation of diallyl sulfide are: biological extraction and chemical synthesis.
- Biological extraction
Process for allicin extraction: fermentation temperature: 50 ~ 55 ℃, fermentation duration: 2h ~ 3h, add water into the garlic with the proportion of 1: 4 water, distilled for the duration of 1.5h ~ 2h.
Organic solvent extraction method is to use organic solvent as extraction agent, and extract the active ingredients of natural products at a certain temperature and extraction duration. Usually methanol, ethanol, acetone, ethyl ether or the mixture of these solvents are used as the organic solvents. Compared withthe steam distillation extraction, the advantage of the organic solvent extraction is that the extract has higher purity. Also it is one of the traditional extraction methods.
Use ethanol with volume fraction of 95% as the extraction solvent, and add the extractant in proportion of 1g: 4mL. First the enzymolysis time was 0.5h when the temperature is set to 40 ℃; then have the extraction for 1.5h when the temperature is set to 30 ℃; finally set the temperature at 50 ℃, and have it concentrated under reduced pressure in a steamer rotating at a speed of 4500r / min to extract allicin. The extraction rate can reach 0.24%.
- Chemical synthesis
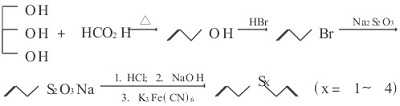
Figure 3: Synthetic route
Uses
Used as a reagent.
Used in the industries such as medical and health, fodder and the like.
Toxicity
GRAS(FEMA)。
Maximum limits
FEMA (mg / kg): Drinks 0.04; Cold Drink 0.06; Confectionery 0.07; Bakery Products 0.05; Seasoning 13; Meat 3.7.
FDA, § 172.515: Appropriate amount.
Chemical Properties
A colorless liquid with characteristic garlic odor. Oil-soluble component of garlic
Chemical Properties
clear colorless liquid
Occurrence
Reported found in garlic (Allium sativum L.), onion (Allium cepa L.), nira (Allium tuberosum Rottl.), caucas (Allium victoralis L.), mustard (Brassica species) and cooked/boiled beef
Uses
antibacterial, antifungal, antineoplastic, antihypercholesterolaemic, hepatoprotectant
Uses
manufacture of flavors.
Uses
Diallyl sulfide is a modulator of drug metabolizing enzyme P450 system and inducer of the phase II detoxifying enzyme GST. Diallyl sulfide is also an inhibitor of CYP2E1 and believed to prevent chemically-induced carcinogenesis in many tissues. It is also used as a food additive. It is also used as an important raw material and intermediate used in organic Synthesis, pharmaceuticals, agrochemicals and dyestuff.
Definition
ChEBI: Diallyl sulfide is an organic sulfide.
Preparation
From allyl iodide plus potassium sulfide in alcoho
Aroma threshold values
Detection at 0.05 ppb
Taste threshold values
Taste characteristics at 2 ppm: sulfureous, onion–garlic and vegetative radish-like with a slightly hot nuance
General Description
Allyl sulfide is a volatile flavor compound naturally found in Allium species such as garlic and onion. It is has a characteristic garlic odor and is used as a flavoring agent in meat and condiments.
Biochem/physiol Actions
Organosulfur compound from garlic that inhibits chemically-induced carcinogenesis in experimental animals. Competitive inhibitor of Cytochrome P450 2E1 (CYP2E1) that, in turn, blocks the activation of several chemcal carcinogens.
Anticancer Research
It is a thioether, found in garlic, inhibits cytochrome P450 IIE1 isoform, and therebysuppresses carcinogenesis (Aggarwal and Shishodia 2004). The consumption ofgarlic provides protection from gastrointestinal cancers and also suppresses theprogression of colorectal adenomas (Hosseini and Ghorbani 2015).
Diallyl sulfide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29884 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 | FandaChem@Gmail.com | China | 9214 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5906 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17365 | 58 |
View Lastest Price from Diallyl sulfide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-11 | Diallyl sulfide
592-88-1
|
US $10.00 / KG | 1KG | 99% | 100 mt | Hebei Weibang Biotechnology Co., Ltd | |
 |
2023-08-24 | Diallyl sulfide
592-88-1
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-02-13 | Allyl Sulfide
592-88-1
|
US $80.00 / kg | 1kg | 99% | 100MT | Hebei baicao biology science and technology co., ltd |
-

- Diallyl sulfide
592-88-1
- US $10.00 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd
-

- Diallyl sulfide
592-88-1
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Allyl Sulfide
592-88-1
- US $80.00 / kg
- 99%
- Hebei baicao biology science and technology co., ltd
592-88-1(Diallyl sulfide)Related Search:
1of4