
Diallyl sulfide synthesis
- Product Name:Diallyl sulfide
- CAS Number:592-88-1
- Molecular formula:C6H10S
- Molecular Weight:114.21
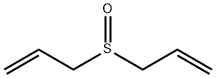
14180-63-3
10 suppliers
inquiry

592-88-1
182 suppliers
$15.00/1g
Yield:592-88-1 90%
Reaction Conditions:
with iodine;sodium hydrogensulfite in chloroform at 50; for 3 h;Temperature;
Steps:
General procedure:
General procedure: A mixture of a sulfoxide (1 mmol), NaHSO3 (1.1 mmol), and I2 (0.025-0.1 mmol) in CHCl3 (1 mL) was stirred at room temperature or at 50 °C. The progress of the reaction was monitored by TLC. After completion, the solution was decanted from the residue, washed with EtOAc (2 x 1 mL). The combined organic layers were concentrated and the residue purified by chromatography on silica gel using n-hexane as eluent.
References:
Abbasi, Mohammad;Mohammadizadeh, Mohammad Reza;Moradi, Zahra [Tetrahedron Letters,2015,vol. 56,# 47,p. 6610 - 6613]

107-05-1
397 suppliers
$16.80/100ML
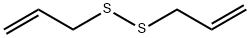
2179-57-9
359 suppliers
$6.00/5g

592-88-1
182 suppliers
$15.00/1g
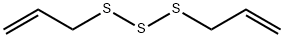
2050-87-5
219 suppliers
$31.00/25mg

107-05-1
397 suppliers
$16.80/100ML
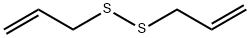
2179-57-9
359 suppliers
$6.00/5g

592-88-1
182 suppliers
$15.00/1g
2050-87-5
219 suppliers
$31.00/25mg

2444-49-7
22 suppliers
$89.00/5mg

6329-40-4
1 suppliers
inquiry

592-88-1
182 suppliers
$15.00/1g
538-74-9
152 suppliers
$21.00/25g
![Benzene, [(2-propen-1-ylthio)methyl]-](/CAS/GIF/6937-97-9.gif)
6937-97-9
0 suppliers
inquiry

106-95-6
414 suppliers
$10.00/5g

592-88-1
182 suppliers
$15.00/1g