
Allyl mercaptan synthesis
- Product Name:Allyl mercaptan
- CAS Number:870-23-5
- Molecular formula:C3H6S
- Molecular Weight:74.14

107-05-1
424 suppliers
$16.80/100ML

870-23-5
147 suppliers
$50.00/1 SAMPLE
Yield:870-23-5 94.19%
Reaction Conditions:
with sodium hydrogen sulfide monohydrate;diallyl disulphide;N-benzyl-N,N,N-triethylammonium chloride in water;xylene at -2 - 40; for 10 h;Product distribution / selectivity;Inert atmosphere;
Steps:
2
Example 2A glass reactor equipped with a reflux condenser, a thermometer, a stirrer and a dropping funnel with a jacket was charged with sodium hydrosulfide hydrate (179.12 g, 1.437 mol) (a sodium hydrosulfide content=45% by weight, a sodium sulfide content=0.50% by weight, and sodium sulfide/sodium hydrosulfide=1.1% by weight), water (35.30 g), xylene (80.00 g), an aqueous solution (55.60 g, 0.170 mol) of 69.6% by weight of triethylbenzylammonium chloride and diallyl disulfide (3.95 g, 0.025 mol), and the resulting mixture was stirred. A nitrogen gas was introduced into a gas-phase layer in the reactor to form a nitrogen gas stream. In this regard, the addition amount of diallyl disulfide is 3.9% by weight based on an addition amount of allyl chloride described later.Then, allyl chloride (100.11 g, 1.282 mol) was charged in the dropping funnel with the jacket and was cooled to a temperature of from -2 to 5° C. The cooled allyl chloride was added dropwise over 7 hours while maintaining a temperature of the reaction solution at 40° C., and then the resulting mixture was further maintained at 40° C. for 3 hours. The content of the diallyl disulfide in the organic phase determined at the start of the heat retention (i.e. zero(0) hour of the heat retention time) was 3.52% by weight. The obtained reaction solution was cooled to a temperature of from 0 to 10° C. and was then admixed with water (120.02 g) so as to dissolve the precipitated sodium chloride. Then, the solution was subjected to oil-water separation to obtain 177.72 g of a solution of allyl mercaptan in xylene as an organic phase. The content of the diallyl disulfide relative to the allyl chloride and the allyl mercaptan in the solution was 3.72% by weight, and the content of the byproduct (4) was 0.02% by weight. The yield of the allyl mercaptan relative to the allyl chloride was 94.19%.
References:
SUMITOMO CHEMICAL COMPANY, LIMITED US2011/218363, 2011, A1 Location in patent:Page/Page column 3-4
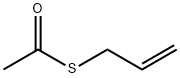
23973-51-5
33 suppliers
inquiry

870-23-5
147 suppliers
$50.00/1 SAMPLE

106-95-6
426 suppliers
$10.00/5g

870-23-5
147 suppliers
$50.00/1 SAMPLE
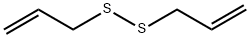
2179-57-9
396 suppliers
$6.00/5g

870-23-5
147 suppliers
$50.00/1 SAMPLE

107-18-6
0 suppliers
$24.60/100ml

870-23-5
147 suppliers
$50.00/1 SAMPLE