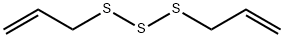
Diallyl trisulfide synthesis
- Product Name:Diallyl trisulfide
- CAS Number:2050-87-5
- Molecular formula:C6H10S3
- Molecular Weight:178.34

870-23-5
140 suppliers
$70.00/2.5g
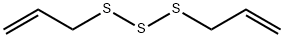
2050-87-5
226 suppliers
$34.00/25mg
Yield:2050-87-5 74%
Reaction Conditions:
Stage #1: prop-2-ene-1-thiolwith triethylamine in propan-1-ol at 20; for 0.333333 h;
Stage #2: with 3,4-dichloro-1,2,5-thiadiazole in propan-1-ol; for 0.666667 h;
Steps:
2.1. General procedure
General procedure: The thiol (5 mmol) and 5.5 mmol of triethylamine (5.5 mmol) were dissolved in 5mL of 1-propanol and stirred for 20min. Then, 3,4-dichloro-1,2,5-thiadiazole (2.5mmol) in 5mL 1-propanol was added dropwise into the flask. The mixture was stirred for the appropriatetime (Table 2) until 3,4-dichloro-1,2,5-thiadiazole was fully consumed (monitored byTLC), and then an aqueous solution of HCl (5 mL) was added, and the mixture stirredfor 20 min. During this time, ethanedinitrile and triethylammonium chloride were dissolvedinto water and the desired trisulfide was easily extracted by the ethyl acetate (3 ×5 mL). The trisulfides were isolated in the 74-85% yields. The pure liquid trisulfides wereobtained after solvent removal. The more pure solid trisulfides could be formed by recrystallizationproducts from the n-hexane and ethyl acetate mixture. The melting points forsolid products are reported in Table 2.Note: due to the toxicity of cyanogen gas, it needs to be trapped as oxamide or stored asgas for future applications.Compounds 2b [30], 2d [52], 2e [51], 2f [53], 2 g [54], 2h [52], 2i [24], and 2k [21]were characterized previously.
References:
Gorjian, Hayedeh;Khaligh, Nader Ghaffari [Journal of Sulfur Chemistry,2022,vol. 43,# 2,p. 169 - 179]

107-05-1
401 suppliers
$16.80/100ML
2179-57-9
369 suppliers
$6.00/5g

592-88-1
184 suppliers
$15.00/1g
2050-87-5
226 suppliers
$34.00/25mg

107-05-1
401 suppliers
$16.80/100ML
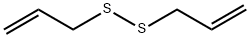
2179-57-9
369 suppliers
$6.00/5g

592-88-1
184 suppliers
$15.00/1g
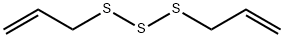
2050-87-5
226 suppliers
$34.00/25mg

2444-49-7
22 suppliers
$97.00/5mg

106-95-6
418 suppliers
$10.00/5g
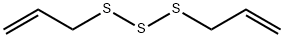
2050-87-5
226 suppliers
$34.00/25mg

107-05-1
401 suppliers
$16.80/100ML
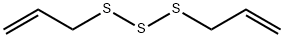
2050-87-5
226 suppliers
$34.00/25mg

2444-49-7
22 suppliers
$97.00/5mg