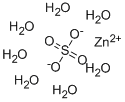
Zinc sulfate heptahydrate
- CAS No.
- 7446-20-0
- Chemical Name:
- Zinc sulfate heptahydrate
- Synonyms
- ZINC SULFATE;ZINC SULPHATE HEPTAHYDRATE;ZINC SULPHATE HEPTA;7446-20-0?;ZINC VITRIOL;ZINC SULFATE HEPTA;Goslarite;VasoClear A;Ophthazinc T;ZINCI SULFAS
- CBNumber:
- CB8854197
- Molecular Formula:
- H14O11SZn
- Molecular Weight:
- 287.56
- MDL Number:
- MFCD00149894
- MOL File:
- 7446-20-0.mol
- MSDS File:
- SDS
| Melting point | 100 °C |
|---|---|
| Density | 1.957 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: soluble100mg/mL, clear, colorless |
| form | Solid |
| Specific Gravity | 1.97 |
| color | White |
| PH | 4-6 (50g/l, H2O, 20℃) |
| Odor | odorless, astringent taste |
| Water Solubility | 960 g/L |
| Sensitive | Hygroscopic |
| Merck | 14,10159 |
| Stability | Stable. Incompatible with strong oxidizing agents. Hygroscopic - protect from moisture. |
| CAS DataBase Reference | 7446-20-0(CAS DataBase Reference) |
| FDA 21 CFR | 182.8997; 582.5997; 182.90; 310.545; 558.258; 582.80 |
| Substances Added to Food (formerly EAFUS) | ZINC SULFATE |
| SCOGS (Select Committee on GRAS Substances) | Zinc sulfate |
| EWG's Food Scores | 3-4 |
| FDA UNII | N57JI2K7WP |
| NCI Dictionary of Cancer Terms | zinc sulfate |
| NCI Drug Dictionary | zinc sulfate |
| ATC code | A12CB01,B05XA18 |
| EPA Substance Registry System | Sulfuric acid, zinc salt (1:1), heptahydrate (7446-20-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H318-H410 | |||||||||
| Precautionary statements | P264-P270-P273-P280-P301+P312-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,N,Xi | |||||||||
| Risk Statements | 22-41-50/53-36/38 | |||||||||
| Safety Statements | 22-26-39-46-60-61-25 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | ZH5300000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28332600 | |||||||||
| Toxicity | LD50 orally in Rabbit: 1260 mg/kg | |||||||||
| NFPA 704 |
|
Zinc sulfate heptahydrate price More Price(52)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 1.08883 | Zinc sulfate heptahydrate for analysis EMSURE? ACS,ISO,Reag. Ph Eur | 7446-20-0 | 500g | $113 | 2024-03-01 | Buy |
| Sigma-Aldrich | 204986 | Zinc sulfate heptahydrate 99.995% trace metals basis | 7446-20-0 | 10g | $75.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.08883 | Zinc sulfate heptahydrate for analysis EMSURE? ACS,ISO,Reag. Ph Eur | 7446-20-0 | 1kg | $177 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.08883 | Zinc sulfate heptahydrate for analysis EMSURE? ACS,ISO,Reag. Ph Eur | 7446-20-0 | 5kg | $612 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.08883 | Zinc sulfate heptahydrate for analysis EMSURE? ACS,ISO,Reag. Ph Eur | 7446-20-0 | 50kg | $5690 | 2024-03-01 | Buy |
Zinc sulfate heptahydrate Chemical Properties,Uses,Production
Outline
At room temperature, zinc sulfate heptahydrate is white particles or powder, it is orthorhombic crystal, it has convergence, it is common astringent, it will weathering in dry air. When be heated 30℃, it can lose a part of crystal water, at 100℃ it loses six crystal water molecules, it loses seven crystal water molecules at 280℃, it decomposes into zinc oxide and sulfur trioxide at 767℃. It is soluble in water, slightly soluble in ethanol and glycerol. It requires confined preservation. It is mainly used as raw material for the production of zinc, barium and other zinc saline, it is also important auxiliary raw material for the viscose fiber and vinylon fiber, it can be also used as mordant dyeing, leather and wood preservative, glue clarifying and preserving agents, medical emetic and fungicides, it can be used for trace element fertilizers on agriculture.
Uses
(1) Zinc sulfate heptahydrate can be used as mordant dyeing, wood preservatives, paper bleaching agent, it can be also used in medicine, synthetic fibers, electrolysis, electroplating, pesticides and production of zinc, etc.
(2) It can be used for the preparation of zinc medicine, astringents, etc.
(3) It can be used as mordant, wood preservatives, bleach paper industry, it is also used in medicine, synthetic fibers, electrolysis, electroplating, pesticides and production of zinc, etc.
(4) Zinc sulfate is zinc supplement of dietary, the component of many enzymes, proteins, such as ribose animals involved in carbohydrate and fat metabolism, and it can catalyze interconversion of pyruvate and lactate, it can promote growth. Zinc deficiency can lead to incomplete keratosis, stunted growth and deterioration of the hair, and it can affect animal reproductive function.
(5) Zinc sulfate is allowed to use in food supplements of zinc. China allows to use it in salt, the used of amount is 500mg /kg; in foods for infants and young children is 113~318mg/kg; in dairy products is 130~250mg/kg; in grains and their products is 80~160rag/kg; in liquid and drink milk drinks is 22.5~44mg/kg.
(6) It is mainly used for man-made fibers coagulating liquid. In printing and dyeing industry, it is used as mordant, salt-stained blue Lamine alkali agent. It is main raw material of manufacturing inorganic pigments (e.g. lithopone), other zinc salts (e.g. zinc stearate, basic zinc carbonate) and zinc-containing catalyst. It is used as wood preservatives and leather, bone glue clarifying and preserving agents. In pharmaceutical industry, it is used as emetic. It can also be used to prevent diseases and fruit tree nurseries and cable manufacturing zinc fertilizer and so on. It can be used as nutritional supplements (zinc enhancer) and the like in food-grade product.
(7) It can be used as analytical reagents, mordant and the phosphor matrix.
Toxicity
Referrence zinc sulfate monohydrate.
Waste battery preparation
The zinc skin of battery is peeled off and washed, then it is put into a beaker, it is cut into small pieces, add water, add concentrated sulfuric acid, properly be heated. If zinc skin dissolves completely, then adds zinc skin, zinc skin is generally control slight excess, this is better handled. Zinc sulfate solution is heated to evaporate, zinc sulfate heptahydrate is crystallized out, it is dried at 120℃, and then dried finished product can be obtained.
Production method
(1) Synthesis method: 18% sulfuric acid was added into to dissolve the dross of dilute solution in reactor, with stirring zinc oxide (or zinc raw material) was added to adjust slurry. Then adds sulfuric acid, the reaction occurs in a stirred acidproof reactor, Ph is controlled at 5.1, it is the end of the reaction, concentration is about 38°Bé. The reaction is filtered, it is heated to 80℃, the addition of zinc to displace copper, cadmium, nickel, then it is filtered, and the filtrate is heated to above 80℃, potassium permanganate (or bleach) is added and heated to boil, the impurity of iron oxide, manganese is oxidated, it is filtered and the filtrate was clarified, then it is evaporated to 49~52 ° Bé, it goes through cooling and crystallization, centrifugal dewatering, drying to obtain zinc sulfate heptahydrate. its
ZnO + H2SO4 → ZnSO4 + H2O
(2) Zinc material is slowly added into diluted sulfuric acid which the relative density is 1.16, temperature is controlled at 80~90℃, after about 2h, Ph value of solution is 5.1 to 5.4, after reaction, the relative density of solution is about 1.35; and then adds small amount of high manganese bleach or potassium, iron, manganese oxide precipitation, filtration spoil the ,filtrate is put into the replacement bucket, then adds small amount of zinc powder and it is stirred at 75~90℃ for 40~50min, replacement of heavy metals copper, lead, cadmium and other impurities. After filtration, the filtrate is oxidated by small amount of bleach or potassium permanganate, further removes small amounts of iron, manganese, and it is filtered to get zinc sulfate solution which relative density is 1.28 to 1.32. This solution is cooled in the crystallizer kettle crystalline for 2~3d, after fractional crystallization, dry finished product is obtained which spin-dried at 40~50℃.
ZnO + H2SO4 → ZnSO4 + H2O
(3) Zinc material is slowly added into diluted sulfuric acid which the relative density is 1.16, temperature is controlled at 80~90℃, after about 2h, Ph value of solution is 5.1 to 5.4, after reaction, the relative density of solution is about 1.35; and then adds small amount of high manganese bleach or potassium, iron, manganese oxide precipitation, filtration spoil the ,filtrate is put into the replacement bucket, then adds small amount of zinc powder and it is stirred at 75~90℃ for 40~50min, replacement of heavy metals copper, lead, cadmium and other impurities. After filtration, the filtrate is oxidated by small amount of bleach or potassium permanganate, further removes small amounts of iron, manganese, and it is filtered to get zinc sulfate solution which relative density is 1.28 to 1.32. This solution is cooled in the crystallizer kettle crystalline for 2~3d, after fractional crystallization, dry finished product is obtained which spin-dried at 40~50℃.
Chemical Properties
Colorless crystals, small needles, or granular, crystalline powder; without odor; astringent, metallic taste. Efflorescent in air. Solutions acid to litmus. Loses 7H2O at 280C. Soluble in water and glycerol; insoluble in alcohol.
Uses
Zinc sulfate heptahydrate is widely used as a coagulant in the production of rayon. It finds an application in the manufacturing of calico printing, wood preservation and lithopone. It is the source of zinc which is supplied in animal feeds, fertilizers and agricultural sprays. It is utilized as electrolytes for zinc plating and surface treating agents. It is also used as a mordant in dyeing, preservative for skin and leather and in medicine as an astringent and emetic.
Uses
Zinc sulfate (ZnSO4?7H2O) is also known as zinc vitriol or white copper. In addition to being used to make rayon, zinc sulfate is used as a wood preservative, a dietary supplement, an animal feed, and as a mordant to prevent dyes from running in printed textiles. It can also be used to stanch bleeding.
Uses
zinc sulfate is a cosmetic astringent and biocide produced through the reaction of sulfuric acid with zinc. It can cause irritation to the skin and mucous membranes, and may cause an allergic reaction.
Uses
Preparation of complexometric titrant
Uses
As mordant in calico-printing; preserving wood and skins; with hypochlorite for bleaching paper; manufacture of lithopone and other zinc salts; clarifying glue; electrodepositing Zn; also as reagent in analytical chemistry.
Definition
ChEBI: A hydrate that is the heptahydrate form of zinc sulfate.
brand name
Verazinc (Forest).
General Description
Zinc sulfate heptahydrate has various industrial applications. It is widely employed as an electrolyte for zinc electroplating and for the storage of solar energy in various solar appliances. Its crystals exhibit diamagnetism.
Agricultural Uses
Zinc sulphate heptahydrate (ZnSO4?7H2O), also called
white vitriol, is the most common form of zinc sulphate.
It forms zinc sulphate monohydrate above 70°C and
anhydrous salt at 280°C, and is used as a mordant and a
styptic (to check bleeding).
Industrial uses
Zinc sulfate (ZnSO4·7H2O) is a white powder, very soluble in water (i.e. 37% at 20 °C). Zinc sulfate is primarily used in base-metal flotation as a depressant for sphalerite. Zinc sulfate has been used to depress talc in by-product molybdenum circuit.
Safety Profile
Human poison by an unspecified route. Poison experimentally by subcutaneous, intravenous, and intraperitoneal routes. Moderately toxic by ingestion. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of SOx and ZnO. See also ZINC SULFATE.
Purification Methods
Crystallise it from aqueous EtOH or dilute H2SO4 below 39o when it forms the heptahydrate, and between 39o and 70o it forms the hexahydrate, and above 70o the monohydrate is stable. The anhydrous salt is obtained from the hydrates by heating at 280o or lower temperatures in a current of dry air. It decomposes to ZnO and SO2 at 767o. The solubility of the heptahydrate in H2O is 5.88% at 0o, 61.92% at 30o, 66.61% at 35o and 70.05% at 39o.
Zinc sulfate heptahydrate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Chongqing Zhihe Biopharmaceutical Co., Ltd. | +8618580541567 | sales@zhswyy.com | China | 303 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20287 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@sdzhonghuimaterial.com | China | 983 | 58 |
| Hangzhou Hyper Chemicals Limited | +86-0086-57187702781 +8613675893055 | info@hyper-chem.com | China | 295 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
View Lastest Price from Zinc sulfate heptahydrate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-25 | Zinc sulfate heptahydrate
7446-20-0
|
US $100.00-75.00 / kg | 1kg | 99% | 5000Ton | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-11-25 | Zinc sulfate heptahydrate
7446-20-0
|
US $1000.00-800.00 / kg | 1kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-11-22 | Zinc Sulfate Heptahydrate
7446-20-0
|
US $75.00-20.00 / kg | 1kg | 99% | 20ton | Hebei Zhuanglai Chemical Trading Co.,Ltd |
-

- Zinc sulfate heptahydrate
7446-20-0
- US $100.00-75.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Zinc sulfate heptahydrate
7446-20-0
- US $1000.00-800.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Zinc Sulfate Heptahydrate
7446-20-0
- US $75.00-20.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd
7446-20-0(Zinc sulfate heptahydrate)Related Search:
1of4