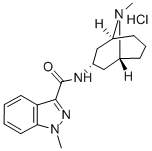
Granisetron Hydrochloride
- CAS No.
- 107007-99-8
- Chemical Name:
- Granisetron Hydrochloride
- Synonyms
- GRANISETRON HYDROCHLORIDE;GRANISETRON HCL;KYTRIL;1-Methyl-N-[(1β,3α,5β)-9-methyl-9-azabicyclo[3.3.1]nonane-3-yl]-1H-indazole-3-carboxamide·hydrochloride;Kitryl;BRL-43694A;Gransetron HCl;GranisetronBase;midehydrochloride;GranisetronHclFda
- CBNumber:
- CB9728851
- Molecular Formula:
- C18H25ClN4O
- Molecular Weight:
- 348.87
- MDL Number:
- MFCD01747034
- MOL File:
- 107007-99-8.mol
- MSDS File:
- SDS
| Melting point | 290-292°C |
|---|---|
| storage temp. | Room temp |
| solubility | H2O: >10mg/mL |
| form | solid |
| color | white to off-white |
| Merck | 14,4536 |
| CAS DataBase Reference | 107007-99-8(CAS DataBase Reference) |
| FDA UNII | 318F6L70J8 |
| NCI Dictionary of Cancer Terms | granisetron hydrochloride |
| NCI Drug Dictionary | granisetron hydrochloride |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P301+P312+P330 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-62-37/38-36/37/38-36 | |||||||||
| Safety Statements | 26-36-37-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | NK7882200 | |||||||||
| HS Code | 29399990 | |||||||||
| NFPA 704 |
|
Granisetron Hydrochloride price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | G3796 | Granisetron hydrochloride ≥98% (HPLC), solid | 107007-99-8 | 10mg | $153 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1298106 | Granisetron hydrochloride United States Pharmacopeia (USP) Reference Standard | 107007-99-8 | 150mg | $974 | 2024-03-01 | Buy |
| TCI Chemical | G0401 | Granisetron Hydrochloride >98.0%(HPLC)(T) | 107007-99-8 | 200mg | $64 | 2024-03-01 | Buy |
| TCI Chemical | G0401 | Granisetron Hydrochloride >98.0%(HPLC)(T) | 107007-99-8 | 1g | $217 | 2024-03-01 | Buy |
| Cayman Chemical | 21239 | Granisetron (hydrochloride) ≥98% | 107007-99-8 | 100mg | $57 | 2024-03-01 | Buy |
Granisetron Hydrochloride Chemical Properties,Uses,Production
Outline of Granisetron hydrochloride
Granisetron hydrochloride was originally developed by the British Beecham company in the mid-1980s. At 1991, the merged Smithkline-Beecham (SB) Company had pushed for the first time of 3 mg of injection Kytri1 to the market in South Africa. To date, including 1 mg of oral tablets, 1mg of injections, granisetron hydrochloride has already entered into market in United States, Britain, France, Japan, Germany, Italy and other more than forty countries and regions around the world. When the Smithkline-Beecham (SB) Company was merged with Glaxo Company, it transferred this product to the Roche Company.
Foreign research data has shown that granisetron hydrochloride has a high selectivity to receptor. Its affinity to 5-HT3 receptor is 4000 to 10000 time as high as its affinity to other receptors, such as 5-HT1, 5-HT2, dopamine D1, D1, histamine H1, benzodiazepine and opioid receptor. For comparison, the difference for ondansetron is only about 1,000 times. For the test of its prevention of cisplatin-induced emesis in ferrets, the antiemetic rates in the three dose groups of granisetron hydrochloride 2 × 0.005,2 × 0.05 and 2 × 0.5mg/kg (iv) were 93%, 96% and 100 %, respectively while for ondansetron in 2 × 2.5mg/kg, this value was 89%, demonstrating that granisetron hydrochloride exhibited a at least five times stronger efficacy of anti-emetic than ondansetron. Toxicity studies suggest that granisetron hydrochloride can achieve very excellent antiemetic effect even in small dose with only minor side effects. However, there may be sth abnormal occurring in cardiovascular system upon large doses. Since the recommended clinical dose of granisetron hydrochloride is small (3mg/d), which is only 1/25 (<1 mg/kg) of the lowest dose used in animal studies, therefore, clinical application of it is very safe. Pharmacokinetic study has shown that granisetron hydrochloride has a half-life (t1/2) of 9 h in the body of patient while this value is 4 h at healthy people, 7.7 h in elderly people and 4.9h in young people.
Granisetron hydrochloride is mainly metabolized in the liver and can be excreted through the feces and urine in the form of 7-hydroxy granisetron hydrochloride and other forms of metabolic product. For patients of liver damage or liver metastases, they have reduced plasma clearance rate with the scavenging rate upon renal insufficiency being 1/4 of the normal case. It has been observed of high first pass metabolism upon oral administration with the absolute bioavailability being 60%.
Domestic Market Analysis
1993, China has approved of the import of the Kytri1 injection (3 mg/ 3 mL) of British SB company, Registration No: X930279, and has allowed for in clinical trials. In 1994, the China-US Tianjin Smith Kline Pharmaceutical Co., Ltd. had used imported raw materials processing method for production of the injection of the 3mg granisetron hydrochloride and had obtained the approval of the Ministry of Health: (94) health medicine approved: J-10 number (fourth class) with the trade name being "Kay Reiter. " Currently there are a number of domestic manufacturers having their products enter into market with the number of manufacture which makes formulation being up to dozen, see Annex I for details.
China has a large population with the annual number of newly identified cancer patients being more than 1.6 million. The nausea and vomiting induced by chemotherapy and radiotherapy-induced have been one of the serious side effects that doctors and patients worry of. Therefore, new antiemetics are in urgent demand. Currently, ondansetron class of drugs has occupied most of the market antiemetic including ondansetron (ShufuLing), granisetron hydrochloride (Kytril) and tropisetron (Oubiting), wherein the ondansetron and granisetron has been accumulated of large number of clinical application experience with good response from the doctors and has played positive role on improving the domestic situation of the treatment of chemotherapy vomiting. With the localization of products, the decreasing price and the increasing income level of people, antiemetics market will be certainly greatly improved. In this case, granisetron hydrochloride, as the secondary antiemetic drug entering into market, will certainly have good market prospects.
The above information is edited by the chemicalbook of Dai Xiongfeng.
Instructions
[Character]it is colorless or nearly colorless liquid clarity.
[Toxicology]This product is a highly selective 5-HT3 receptor antagonist and has excellent preventive and therapeutic effect on treating nausea and vomiting induced by radiotherapy, chemotherapy and surgery. Radiotherapy, chemotherapy and surgery and other factors can cause enterochromaffin cells to release 5-HT; 5-HT can activate the 5-HT3 receptors in central nerve or vagus nerve and cause vomiting reflex. The mechanism of this product in controlling nausea and vomiting is through antagonizing the 5-HT3 receptor in the central chemosensory area and peripheral vagus nerve terminal, further suppressing the nausea and vomiting. This product has high selectivity and no side effects such as extrapyramidal reactions, excessive sedation and so on.
[Indications]it is suitable for the treatment of nausea and vomiting induced by radiation therapy and cytotoxic drug.
[Dosage and usage amount]intravenous drip. Recommended dosage for adults is usually 3 mg. Administer it intravenously 30 minutes prior to chemotherapy. Most patients only need to be administered once with the prevention action against nausea and vomiting being more than 24 hours. The number of doses can be increased by 1-2 times if necessary, but the maximum daily dose should not exceed 9 mg and the infusion time should not be less than 5 minutes.
[Pharmacokinetics]healthy volunteers, after intravenous injection of this product for 20 or 40 μg/kg, the average peak plasma concentration was 13.7 and 42.8μg/L, the plasma elimination half-life is approximately 3.1-5.9 hours. This product is widely distributed in the body with serum protein binding rate being about 65%. Most of them are rapidly metabolized with the major metabolic pathway being N-dealkylation and conjugation after aromatic oxidation. This product is excreted through the feces and urine.
Chemical Properties
It is white or yellowish white crystalline powder and is odorless. It is easily soluble in water but hardly soluble in methanol, extremely hardly to be dissolved in ethanol and almost insoluble in ether. It has a melting point of 290-292 ℃. For acute toxicity LD50, female mice, male and female rats (mg/kg): 17, 25, 14, 16, respectively. Intravenous injection.
Uses
It is the selective antagonist of the 5-serotonin 3 (5-HT3) in peripheral and central nervous system.
Description
Granisetron hydrochloride is the second selective 5HT-3-antagonist approved for the management of nausea and vomiting induced by cancer chemotherapy.Like ondansetron, granisetron hydrochloride is superior to metoclopramide in both efficacy and side effect profile. The compound appears to be effective both as a prophylactic agent and in blocking vomiting once started.
Chemical Properties
White to Off-White Crystalline Sold
Originator
SmithKline Beecham (United Kingdom)
Uses
anticoagulant
Uses
A specific serotonin (5HT3) receptor antagonist. Used as an antiemetic. Granisetron HCl is a serotonin 5-HT3 receptor antagonist used as an antiemetic to treat nausea and vomiting following chemothera py. Its main effect is to reduce the activity of the vagus nerve. It does not have much effect on vomiting due to motion sickness. This drug does not have any effect on dopamine receptors or muscarini c receptor
Uses
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Manufacturing Process
A solution of 1-(phenylmethyleneamino)isatin (1.354 g, 0.0054 mol) and
endo-3-amino-9-methyl-9-azabicyclo[3.3.1]nonane (0.832 g, 0.0054 mol) in
dry THF (25 ml) under argon was heated to reflux for 5 hours. The solution
was cooled and evaporated and the residual traces of THF were azeotropically
removed with dichloromethane. The residue was triturated with ether to give
the intermediate 2-(benzylidenehydrazino)-α-oxophenyl-(9-methyl-9-
azabicyclo[3.3.1]non-3-yl)carboxamide as an orange powder (1.583 g, 73%).
Sodium hydride (50 mg, 60% dispersion in oil) was added to a solution of the
2-(benzylidenehydrazino)-α-oxophenyl-(9-methyl-9-azabicyclo[3.3.1]non-3-yl)
carboxamide in dry THF (2.3 ml) under argon at -50°C. The resultant solution
was warmed to 0°C over 20 min then cooled to -30°C and treated with methyl
iodide (0.020 ml). The solution was allowed to warm to room temperature and
stirred for 24 hours then filtered. The filtrate was evaporated to dryness, and
triturated with chloroform to give the 2-(N-methylbenzylidenehydrazo)-α-
oxophenyl-(9-methyl-9-azabicyclo[3.3.1]non-3-yl)carboxamide as a light
coloured powder (50 mg, 38%). Further trituration of the mother liquor gave
a further crop, (37 mg, 28%, after recrystallisation from chloroform).
A solution of 2-(N-methylbenzylidenehydrazo)-α-oxophenyl-(9-methyl-9-
azabicyclo[3.3.1]non-3-yl)carboxamide (37 mg) in methanol (1 ml) was
treated with 2 N hydrochloric acid (0.1 ml) and left at room temperature for
several hours. Evaporation of the solvent gave the crude product as a brown
oil (36 mg). HPLC and MS analysis confirmed the structure and indicated a
quantitative yield of endo-N-(9-methyl-9-azabicyclo[3.3.1]non-3-yl)-1-
methylindazole-3-carboxamide (Ganisetron).
In practice it is usually used as hydrochloride.
brand name
Kytril (Roche).
Therapeutic Function
Serotonin antagonist
General Description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Biological Activity
5-HT 3 receptor antagonist that possesses potent antiemetic activity.
Biochem/physiol Actions
Granisetron hydrochloride is a serotonin 5-HT3 receptor antagonist and antiemetic.
Pharmacology
Granisetron Hydrochloride is a highly selective 5-HT3 receptor antagonist with good preventive and therapeutic effects on nausea and vomiting caused by radiotherapy, chemotherapy and surgery. Factors such as radiotherapy, chemotherapy and surgery can cause enterochromaffin cells to release 5-HT, and 5-HT can activate 5-HT3 receptors in the central or vagus nerve and cause gag reflex. The mechanism of this product to control nausea and vomiting is to inhibit the occurrence of nausea and vomiting by antagonizing the 5-HT3 receptors in the central chemoreceptor area and peripheral vagus nerve endings.
Pharmacokinetics
After intravenous injection of 20 or 40 μg/kg of this product in healthy subjects, the average peak plasma concentration was 13.7 and 42.8 μg/L, and the plasma elimination half-life was about 3.1-5.9 hours. Granisetron hydrochloride is widely distributed in the body, the serum protein binding rate is about 65%, and most of it is rapidly metabolized. The main metabolic pathway is N-dealkylation and aromatic epoxidation and then conjugated. It is excreted in feces and urine.
Veterinary Drugs and Treatments
Granisetron is an alternative to other 5-HT3 receptor antagonists (e.g., ondansetron or dolasetron) for the treatment of severe vomiting or prophylaxis before administering antineoplastic drugs such as cisplatin that can cause severe vomiting.
storage
Desiccate at +4°C
Granisetron Hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12841 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1803 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29881 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
View Lastest Price from Granisetron Hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-29 | Granisetron Hydrochloride/1-Methylindazole-3-carboxylic acid
107007-99-8
|
US $0.00 / Kg/Bag | 100g | 97%-102% HPLC; EP | 10KGS | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-29 | Granisetron Hydrochloride
107007-99-8
|
US $0.00 / Kg/Bag | 2Kg/Bag | 99% up, High Density | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-11-29 | Granisetron Hydrochloride
107007-99-8
|
US $0.00 / Kg/Bag | 2Kg/Bag | 99% up, High Density | 20 tons | Sinoway Industrial co., ltd. |
-

- Granisetron Hydrochloride/1-Methylindazole-3-carboxylic acid
107007-99-8
- US $0.00 / Kg/Bag
- 97%-102% HPLC; EP
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Granisetron Hydrochloride
107007-99-8
- US $0.00 / Kg/Bag
- 99% up, High Density
- Sinoway Industrial co., ltd.
-

- Granisetron Hydrochloride
107007-99-8
- US $0.00 / Kg/Bag
- 99% up, High Density
- Sinoway Industrial co., ltd.
107007-99-8(Granisetron Hydrochloride)Related Search:
1of4