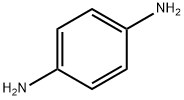
p-Phenylenediamine
- CAS No.
- 106-50-3
- Chemical Name:
- p-Phenylenediamine
- Synonyms
- BENZENE-1,4-DIAMINE;1,4-DIAMINOBENZENE;PARA PHENYLENE DIAMINE;1,4-PHENYLENEDIAMINE;1,4-BENZENEDIAMINE;1,4-benzendiamine;4-phenylenediamine;p-phenylene;JAROCOL PPD;4-AMINOANILINE
- CBNumber:
- CB9852680
- Molecular Formula:
- C6H8N2
- Molecular Weight:
- 108.14
- MDL Number:
- MFCD00007901
- MOL File:
- 106-50-3.mol
- MSDS File:
- SDS
| Melting point | 138-143 °C (lit.) |
|---|---|
| Boiling point | 267 °C (lit.) |
| Density | 1.135 g/cm3 (20℃) |
| vapor density | 3.7 (vs air) |
| vapor pressure | 1.08 mm Hg ( 100 °C) |
| refractive index | 1.6339 (estimate) |
| Flash point | 156 °C |
| storage temp. | Store below +30°C. |
| solubility | Soluble in alcohol, chloroform, ether and hot benzene. |
| form | Powder or Flakes |
| pka | 4.17(at 25℃) |
| Colour Index | 76060 |
| color | White, gray, or purple to brown |
| PH Range | NonQ uorescence (3.1) to orange/yellow Q uorescence (4.4) |
| PH | 9 (50g/l, H2O, 20℃) |
| Water Solubility | 47 g/L (25 ºC) |
| Merck | 14,7285 |
| BRN | 742029 |
| Exposure limits | TLV-TWA 0.1 mg/m3 (ACGIH 1989); TWA skin 0.1 mg/m3 (MSHA and OSHA); IDLH 25 mg/m3 (NIOSH); carcinogenicity: Animal Inadequate Evidence (IARC). . |
| Stability | Stable, but oxidizes when exposed to air. Incompatible with oxidizing agents. Store under inert atmosphere. |
| Major Application | Nanoparticles, liquid crystal displays, chemical mechanical polishing, bottom antireflective coatings, electrochromic materials, inks, rubber, hair dyes, cosmetics, treatment of virus skin infection |
| InChIKey | CBCKQZAAMUWICA-UHFFFAOYSA-N |
| LogP | -0.84 at 20℃ |
| Indirect Additives used in Food Contact Substances | P-PHENYLENEDIAMINE |
| CAS DataBase Reference | 106-50-3(CAS DataBase Reference) |
| EWG's Food Scores | 5-7 |
| FDA UNII | U770QIT64J |
| IARC | 3 (Vol. 16, Sup 7) 1987 |
| NIST Chemistry Reference | 1,4-Benzenediamine(106-50-3) |
| EPA Substance Registry System | p-Phenylenediamine (106-50-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311+H331-H317-H319-H370-H410 | |||||||||
| Precautionary statements | P273-P280-P301+P310-P302+P352+P312-P304+P340+P311-P305+P351+P338 | |||||||||
| Hazard Codes | T,N,T+,Xn | |||||||||
| Risk Statements | 23/24/25-36-43-50/53-63-36/37/38-45-40-48/22-67-52/53 | |||||||||
| Safety Statements | 28-36/37-45-60-61-28A-24/25-23-53-26 | |||||||||
| OEB | D | |||||||||
| OEL | TWA: 0.1 mg/m3 [skin] | |||||||||
| RIDADR | UN 1673 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | SS8050000 | |||||||||
| F | 8-10-23 | |||||||||
| Autoignition Temperature | 567 °C | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29215119 | |||||||||
| Toxicity | LD50 in rats (mg/kg): 80 orally, 37 i.p. (Burnett) | |||||||||
| IDLA | 25 mg/m3 | |||||||||
| NFPA 704 |
|
p-Phenylenediamine price More Price(30)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.07246 | 1,4-Phenylenediamine for synthesis | 106-50-3 | 5G | $31.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.07246 | 1,4-Phenylenediamine for synthesis | 106-50-3 | 250g | $51.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.07246 | 1,4-Phenylenediamine for synthesis | 106-50-3 | 1kg | $154 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.07246 | 1,4-Phenylenediamine for synthesis | 106-50-3 | 25kg | $2130 | 2024-03-01 | Buy |
| Sigma-Aldrich | 695106 | p-Phenylenediamine anti-fade reagent | 106-50-3 | 1g | $52 | 2024-03-01 | Buy |
p-Phenylenediamine Chemical Properties,Uses,Production
Description
P-phenylenediamine is one of the simplest aromatic diamine with the pure product being white to purple red crystals. It turns purple or dark brown color when being exposed to the air. It is slightly soluble in cold water, soluble in alcohol, ether, chloroform and benzene. It can be used for making azo dyes, high-molecule polymers and can also be used for the production of fur dyes, rubber antioxidants and photo developer and is mainly used for Kevlar, azo dyes, sulfur dyes, acid dyes as well as being used for the production of black fur D, black fur DB, brown fur N2 as well as rubber antioxidant DNP, DOP and MB. It can also be used as the raw material of cosmetic hair p-Phenylenediamine series, gasoline polymerization inhibitor and developer. P-phenylenediamine, as a chemical dye, is currently permitted for use in hair dye production, but there is a clear usage limits. According to China "Hygienic Standard for Cosmetics," the content of p-phenylenediamine in hair dyes should not exceed 6%. According to the introduction of experts, although five kinds of "Yixihei" shampoo have their p-phenylenediamine content being within 1.1% to 1.4%. However, the shampoo has high frequency of usage with long-term accumulation being prone to pose a threat to the health and safety of consumers. There is still no literatures regarding to whether phenylenediamine is carcinogenic or not; but there is literature basis regarding to that p-phenylenediamine drug is toxic organic. We can refer to Shanghai Science and technology press (November 1985) "reagent Handbook" (second edition), page 980. Overseas research has shown that for population being subject to frequent hair dying has the incidence of breast cancer, skin cancer and leukemia increased. In addition, p-phenylenediamine is also a common sensitive reagent for testing iron and copper. In the international arena, it is also used for aircraft coatings, bullet-proof clothing intima and walls paint.
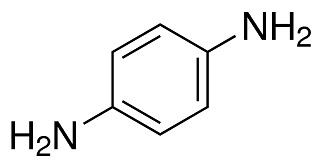
p-phenylenediamine structure
Chemical Properties
It is white to purple red crystal. It will turn purple red or dark brown when exposure to the air. It can be dissolved in water, alcohol, ether, chloroform and benzene.
Uses
P-phenylenediamine is important dye intermediates and is mainly used in the manufacture of dyes and sulfur dyes; it can also be used for the production of fur black D and rubber antioxidant DNP, 288, DOP, DBP etc. P-phenylenediamine can also be used as the raw material of cosmetic hair dye “WuErsi D”, gasoline polymerization inhibitor and developer.
Production method
It can be obtained from the reduction of P-nitroaniline via iron in acid medium. Put the iron into hydrochloric acid and heat to 90 °C. Add with stirring of p-nitroaniline. After completion of the adding, have them reacted at 95-100 ℃ for 0.5h, and then add drop wise of concentrated hydrochloric acid so that the reduction reaction is completed. After cooling, use saturated sodium carbonate solution for neutralizing to PH7-8, after boiling and have hot filtration; use host water to wash the filter cake. The filtrate and washings were combined and subject to concentration under reduced pressure; after cooling crystallization or vacuum distillation, we can obtain the p-phenylenediamine with the yield of being 95%.
Acute toxicity
Oral-rat LD50: 80 mg/kg; oral-mouse LDL0: 100 mg/kg.
Irritation data
Skin-rabbit 250 mg/24 hours, moderate.
Hazardous characteristics of explosive
It is explosive when mixed with air.
Flammability and hazard characteristics
It is combustible upon fire, heat and oxidants with combustion releasing toxic fumes of nitrogen oxides.
Storage characteristics
Treasury: ventilation, low-temperature and dry; store it separately from oxidants and food additives.
Description
Paraphenylenediamine (PPD) is a colorless compound oxidized by hydrogen peroxide in the presence of ammonia. It is then polymerized by a coupling agent to produce a color.
Chemical Properties
p-Phenylenediamines are white to slightly red crystalline solids. They have been described as gray “light brown” which may result from exposure to air.
Physical properties
White, red, or brown crystals. May darken on exposure to air.
Uses
4-Phenylenediamine is an azo-dye intermediate; photographic-developing agent; photo-chemical measurements; intermediate in manufacture of antioxidants and accelerators for rubber; laboratory reagent; dye for hair and fur; lithography; photocopying; oils; greases; gasoline; antioxidant/accelerator in the rubber and plastic industry; the hydrochloride is used as blood reagent.
Uses
p-Phenylenediamine is used for dyeing hairand fur, in the manufacture of azo dyes, inaccelerating vulcanization of rubber, and inantioxidants.
Uses
A hair dye component, paraphenylenediamine, as a contact allergen for treatment of inflammatory diseases.
Definition
ChEBI: A phenylenediamine in which the amino functions are at positions 1 and 4 of the benzene nucleus.
General Description
A white to purple crystalline solid (melting point 234 F) that turns purple to black in air. Flash point 309 F. Toxic by skin absorption, inhalation or ingestion. Used for production of aramid fiber, antioxidants, as a laboratory reagent, in photographic developing, and as a dye for hair and furs.
Air & Water Reactions
Oxidizes on exposure to air. The finely powdered base if suspended in air poses a significant dust explosion hazard. Soluble in water. Even as a solid will spot downwind areas purple/black (Roger Patrick, DuPont Engineer).
Reactivity Profile
p-Phenylenediamine is the stongest of the weak aromatic bases. p-Phenylenediamine neutralizes acids in weak exothermic reactions to form salts. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Reacts readily with oxidizing agents .
Health Hazard
p-Phenylenediamine is a moderate to highlytoxic compound; the acute, subacute andchronic toxicity of this amine is greater thanthat of its ortho- and meta-isomers. The acutepoisoning effects in animals were manifestedby lacrimation, salivation, ataxia, tremor,lowering of body temperature, increasedpulse rate, and respiratory depression. Anintraperitoneal dose of 10.8 mg/kg (sus pended in propylene glycol) in male ratscaused the formation of methemoglobin tothe extent of 12.9% after 5 hours (Watan abe et al. 1976). The hydrochloride of thisamine has been reported to cause edemaof the head and neck in animals dosedwith 120–350 mg/kg. p-Phenylenediamine in hair dye formulations produced skinirritation and mild conjunctivial inflamma tion in a variety of test animals (Lloydet al. 1977). In guinea pigs, contact pro duced skin sensitization. Hair dyes con taining p-phenylenediamine damaged visionwhen applied into eyes. In addition, allergicasthma and inflammation of the respiratorytract resulted from exposure to higher con centrations. Reports in early literature citeseveral cases of human poisoning resultingfrom the use of hair dyes containingp-phenylenediamine. The toxic symptomsreported were liver and spleen enlargement,vertigo, gastritis, jaundice, atrophy of liver,allergic asthma, dermatitis, cornea ulcer,burning and redness in eyes, and presbyopia(the latter effects arising from using hair dyeson the eyebrows and eye lashes).
Tests for mutagenicity in Salmonellamicrosome assays (in vitro) were negative.With metabolic activation, upon oxidationwith hydrogen peroxide, most mutagenictests showed positive results. Tests forcarcinogenicity were negative, although itslightly increased the overall tumor ratein experimental animals. After oxidationwith hydrogen peroxide, the amine producedtumors in the mammary glands of female rats(Rojanapo et al. 1986).
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
p-Phenylenediamine causes allergic reactions with skin. It is widely used in hair dyes. It eventually forms Bandrowski′s base, which is found to be the primary cause for allergy. p-Phenylenediamine exposure results in dermatitis, urticaria and anaphylaxis. It acts as an electron donor and is known to reduce cytochrome c.
Contact allergens
PPD is a colorless compound oxidized by hydrogen peroxide in the presence of ammonia. It is then polymerized to a color by a coupling agent. Although a wellknown allergen in hair dyes, PPD can be found as a cause of contact dermatitis in chin rest stains or in milk testers. It is also a marker of group sensitivity to para amino compounds such as benzocaine, some azo dyes, and some previous antibacterial sulphonamides.
Safety Profile
Suspected carcinogen with experimental tumorigenic data. Poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Mildly toxic by skin contact. A human skin irritant. Mutation data reported. Implicated in aplastic anemia, Can cause fatal liver damage. The p-form is more toxic and a stronger irritant than the 0and misomers. Wen used as a hair dye it caused vertigo, anemia, gastritis, exfoliative dermatitis, and death. Has caused asthma and other respiratory symptoms in the fur-dyeing industry. Combustible when exposed to heat or flame; can react vigorously with oxidizing materials. To fight fire, use water, Con, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also other phenylenediamine entries and AMINES
Potential Exposure
p-Phenylenediamine has been used in dyestuff manufacture, in hair dyes; in photographic developers; in synthetic fibers; polyurethanes, and as a monomer and in the manufacture of improved tire cords. Also used as a gasoline additive and in making antioxidants.
Carcinogenicity
A number of dermal carcinogenesis bioassays have been
reported using p-PDA alone in an organic solvent or in
combination with hydrogen peroxide. An 85-week study
in which female Swiss mice were treated with 5% or 10%
p-PDAin acetone, 0.02 mL/animal applied topically, showed
no evidence of carcinogenicity.
p-PDA was not found to be carcinogenic when administered
by diet to male and female F344 rats and B6C3F1 mice
at the dietary doses of 625 or 1250 ppm; the high dose
approximated the maximum tolerated dose. An
IARC Working Group concluded that on the basis of lack
of human data, and inadequate animal data, p-PDA was not
classifiable as to its carcinogenicity to humans. A
recent meta-analysis of 11 case-control studies and one
cohort study of the relationship between p-PDA exposure
through use of personal hair dye and bladder cancer did not
indicate any causal association.
Source
Bulk quantitities may contain m- and o-phenylenediamine and aniline as impurities.
Environmental Fate
Biological. In activated sludge, 3.8% mineralized to carbon dioxide after 5 d (Freitag et al.,
1985). In activated sludge inoculum, following a 20-d adaptation period, 80.0% COD removal was
achieved (Pitter, 1976).
Photolytic. A carbon dioxide yield of 53.7% was achieved when phenylenediamine (presumably
an isomeric mixture) adsorbed on silica gel was irradiated with light (λ >290 nm) for 17 h (Freitag
et al., 1985).
Chemical/Physical. p-Phenylenediamine will not hydrolyze because it does not contain a
hydrolyzable functional group (Kollig, 1993).
Shipping
UN1673 Phenylenediamines (o-, m-, p-), Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Crystallise the diamine from EtOH or *benzene, and sublime it in vacuo; protect it from light. The acetate has m 304o. [Beilstein 13 IV 104.]
Incompatibilities
Dust may form explosive mixture with air. A strong reducing agent. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, acid chlorides; acid anhydrides; chloroformates, and strong bases. Incompatible with organic anhydrides; isocyanates, aldehydes. Heat and light contribute to instability. Keep away from metals.
Waste Disposal
Controlled incineration whereby oxides of nitrogen are removed from the effluent gas by scrubber, catalytic or thermal device.
p-Phenylenediamine Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 | dominicguo@gk-bio.com | CHINA | 9414 | 58 |
| Shandong Zhishang New Material Co., Ltd. | +8617653113209 | sales002@sdzschem.com | China | 3049 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418684 +8618949823763 | sales@tnjchem.com | China | 25356 | 58 |
| Qingdao Scienoc Chemical CO Ltd | +86-0532-68076095 +8618661673503 | info@scienoc.com | China | 113 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615350571055 | Sibel@weibangbio.com | China | 6086 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Hebei Dangtong Import and export Co LTD | +86-13910575315 +86-13910575315 | admin@hbdangtong.com | China | 1000 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20285 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
Related articles
- The introduction of p-Phenylenediamine
- PPD has a strong protein binding ability to penetrate deeply into the hair. It is widely used as an oxidizable hair dye for it....
- May 14,2024
- P-phenylenediamine Side effect
- Acute (short-term) exposure to high levels of p-phenylenediamine may cause severe dermatitis, eye irritation and tearing, asth....
- Mar 6,2023
View Lastest Price from p-Phenylenediamine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-29 | p-Phenylenediamine
106-50-3
|
US $0.00 / KG | 1KG | 99.9%min | 10000kg/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-29 | p-Phenylenediamine (PPD)
106-50-3
|
US $1.00 / KG | 1KG | ≥99% | 5000mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2024-10-25 | p-Phenylenediamine
106-50-3
|
US $100.00 / kg | 1kg | 99%min | 200TON | Hebei Weibang Biotechnology Co., Ltd |
-

- p-Phenylenediamine
106-50-3
- US $0.00 / KG
- 99.9%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- p-Phenylenediamine (PPD)
106-50-3
- US $1.00 / KG
- ≥99%
- Jinan Finer Chemical Co., Ltd
-

- p-Phenylenediamine
106-50-3
- US $100.00 / kg
- 99%min
- Hebei Weibang Biotechnology Co., Ltd