ChemicalBook > Product Catalog >Inorganic chemistry >Inorganic salts >Metal Sulphides and sulphates >Tin sulfide
Tin sulfide
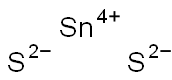
- CAS No.
- 1315-01-1
- Chemical Name:
- Tin sulfide
- Synonyms
- Mosaic gold;indisulfide;TIN SULFIDE;TinDisulphide;STANNIC SULFIDE;tinsulfide(sns2);TIN (IV) SULFIDE;Einecs 215-252-9;Tin(IV)disulfide;Stannic sulphide
- CBNumber:
- CB0143370
- Molecular Formula:
- S2Sn
- Molecular Weight:
- 182.84
- MOL File:
- 1315-01-1.mol
- Modify Date:
- 2024/7/9 8:55:10
| Melting point | decomposes at 600℃ [HAW93] |
|---|---|
| Boiling point | 600°C |
| Density | 4,5 g/cm3 |
| vapor pressure | 0Pa at 25℃ |
| solubility | insoluble in H2O; soluble in alkaline solutions, aquaregia |
| form | gold-yellow hexagonal crystals |
| color | gold-yellow hexagonal, hexane crystals, crystalline |
| Water Solubility | insoluble H2O, dilute mineral acids; soluble aqua regia, alkali hydroxide solutions [MER06] |
| CAS DataBase Reference | 1315-01-1 |
| EPA Substance Registry System | Tin sulfide (SnS2) (1315-01-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS02 |
|---|---|
| Signal word | Danger |
| Hazard statements | H304-H225 |
| Precautionary statements | P501-P240-P210-P233-P243-P241-P242-P280-P370+P378-P331-P303+P361+P353-P301+P310-P403+P235-P405 |
| Hazard Codes | T |
| Risk Statements | 20/21/22-36/37/38 |
| Safety Statements | 26-36/37/39 |
| HS Code | 2830908500 |
Tin sulfide price
Tin sulfide Chemical Properties,Uses,Production
Chemical Properties
Yellow to brown powder. Soluble in concentrated hydrochloric acid and alkaline sulfides; insoluble in water. Flaky or scaly crystals with a high gold-yellow sheen, soft as talc; very stable in air. On heating, the color deepens reversibly to dark red, then to deep brown, above about 600°C decomposition to SnS and S takes place.
Uses
Gilding and bronzing metals, gypsum, wood and paper, usually by suspending in lacquer or varnish.
Hazard
Irritant to skin and eyes
Synthesis
Tin sulfide can be prepared as a bright yellow precipitate by the action of hydrogen sulfide on tin chloride solution.
Tin sulfide can also be prepared by the reaction of the metal element tin with monomeric sulphur.
Sn + 2 S = SnS2
Tin sulfide Preparation Products And Raw materials
Raw materials
Preparation Products
Global( 108)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Kindchem(Nanjing)Co.,Ltd | +86-025-025-85281586 +8618651653755 | China | 1227 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29899 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 | China | 3005 | 58 | Inquiry |
| changzhou huayang technology co., ltd | +8615250961469 | China | 9821 | 58 | Inquiry |
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 | China | 2739 | 58 | Inquiry |
| Alfa Chemistry | +1-5166625404 | United States | 21317 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 9003 | 58 | Inquiry |
| LEAP CHEM CO., LTD. | +86-852-30606658 | China | 24738 | 58 | Inquiry |
1315-01-1(Tin sulfide)Related Search:
chevron_left
1of4
chevron_right
STANNIC SULFIDE
TIN (IV) SULFIDE
TinDisulphide
tinsulfide(sns2)
Mosaic gold
Pigment yellow 38 (C.I. 77878)
Tin (IV) sulfide, 99.5% (metals basis)
Tin(IV)disulfide
Einecs 215-252-9
Tin(IV) sulphide 99.9%
Tin sulfide (SnS2 SnS2)
Tin(IV)sulfide(SnS2)
indisulfide
Tin (IV) Sulfide, 99.99%
Stannic sulphide
TIN SULFIDE
Samarium Oxide (Sm2O3) Sputtering Targets
1315-01-1
SnS2
2SSn





