1-Naphthol
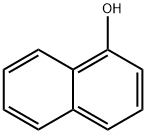
- CAS No.
- 90-15-3
- Chemical Name:
- 1-Naphthol
- Synonyms
- naphthalen-1-ol;1-naphthalenol;1-HYDROXYNAPHTHALENE;ALPHA-NAPHTHOL;α-Naphthol;α-Naphthol;1-naphtol;ALPHA-NAPTHOL;furro er;Oxidation base 33
- CBNumber:
- CB0279325
- Molecular Formula:
- C10H8O
- Molecular Weight:
- 144.17
- MOL File:
- 90-15-3.mol
- Modify Date:
- 2024/5/29 17:03:55
| Melting point | 94-96 °C(lit.) |
|---|---|
| Boiling point | 278-280 °C(lit.) |
| Density | 1.224 |
| vapor density | 4.5 (120 °C, vs air) |
| vapor pressure | 1 mm Hg ( 94 °C) |
| refractive index | 1.6224 |
| Flash point | 125 °C |
| storage temp. | Store below +30°C. |
| solubility | Soluble in benzene, chloroform, ether and ethanol. |
| pka | 9.34(at 25℃) |
| form | Crystalline Flakes |
| color | white to off-white |
| Odor | Slight phenolic odor |
| explosive limit | 5% |
| Water Solubility | 436.7mg/L(25 ºC) |
| λmax | 324nm(MeOH)(lit.) |
| Sensitive | Air & Light Sensitive |
| Merck | 14,6383 |
| BRN | 1817321 |
| Stability | Stable, but air and light sensitive - store under inert gas. Incompatible with strong bases, strong oxidizing agents. |
| InChIKey | KJCVRFUGPWSIIH-UHFFFAOYSA-N |
| LogP | 2.84 at 25℃ |
| CAS DataBase Reference | 90-15-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Naphthalenol(90-15-3) |
| EPA Substance Registry System | 1-Naphthol (90-15-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS05,GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H311-H315-H317-H318-H335-H371-H410 | |||||||||
| Precautionary statements | P273-P280-P301+P312-P302+P352+P312-P305+P351+P338-P308+P311 | |||||||||
| Hazard Codes | Xn,N | |||||||||
| Risk Statements | 21/22-37/38-41-51/53 | |||||||||
| Safety Statements | 22-26-37/39-2-61 | |||||||||
| RIDADR | UN 2811 6.1/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | QL2800000 | |||||||||
| F | 8-23 | |||||||||
| Autoignition Temperature | 510 °C | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29071510 | |||||||||
| Toxicity | LD50 orally in Rabbit: 1870 mg/kg LD50 dermal Rabbit 880 mg/kg | |||||||||
| NFPA 704 |
|
1-Naphthol price More Price(32)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | N2780 | 1-Naphthol BioXtra, ≥99% | 90-15-3 | 10G | ₹7339.35 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | N2780 | 1-Naphthol BioXtra, ≥99% | 90-15-3 | 100G | ₹34022.98 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | N1000 | 1-Naphthol ReagentPlus?, ≥99% | 90-15-3 | 10G | ₹3626.38 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | N1000 | 1-Naphthol ReagentPlus?, ≥99% | 90-15-3 | 100G | ₹8465.15 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | N1000 | 1-Naphthol ReagentPlus?, ≥99% | 90-15-3 | 500G | ₹17753 | 2022-06-14 | Buy |
1-Naphthol Chemical Properties,Uses,Production
Description
1-Naphthol, or α-naphthol, is a fluorescent organic compound with the formula C10H7OH. It is a white solid. It is an isomer of 2-naphthol differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, with the hydroxyl group being more reactive than in the phenols. Both isomers are soluble in simple alcohols, ethers, and chloroform. They are precursors to a variety of useful compounds. Naphthols (both 1 and 2 isomers) are used as biomarkers for livestock and humans exposed to polycyclic aromatic hydrocarbons.
α-naphtol, combined with epichlorhydrine and sodium hydroxide to form alpha-naphtyl glycidyl ether, caused sensitization in one of three workers in a chemical plant.
Chemical Properties
Pale grey to brown solid with an unpleasant phenol odour. Darkens in the presence of light. Evaporates with water vapour. Soluble in ethanol, ether, benzene, chloroform and alkali solutions, insoluble in water. Precipitates violet when exposed to ferric chloride. 1-Naphthol [90-15-3] a-naphthol, 1- naphthalenol, 1-hydroxynaphthalene, C10H8O, Mr 144.16, forms colorless prisms (from toluene) which darken on exposure to air or light. The compound is steam volatile and sublimable. 1-Naphthol is reduced by sodium in liquid ammonia to give 5,6,7,8-tetrahydro-1-naphthol and is oxidized by NaOCl – FeCl3 or I2–KI to give a violet color. It is chlorinated by phosphorus pentachloride at 150℃ to give 1-chloronaphthalene, by sulfuryl chloride to give 4- chloro-1-naphthol, or by Cl2–CH3COOH to give 2,4-dichloro-1-naphthol. Similarly bromine forms 2,4-dibromo-1-naphthol, and this reaction may be used quantitatively for titration. Nitration of 1-naphthol gives complex mixtures. The crude 2,4-dinitro derivative was used in the mid-1800s as an acid yellow dye comparable to picric acid. Sulfonation at 50℃ readily yields 1-naphthol-2,4-disulfonic acid. Nitrous acid gives mainly the 2-nitroso derivative, contaminated with the 4-nitroso isomer.
Uses
1-Naphthol is used as a precursor in the manufacturing of various azo dyes and pharmaceuticals such as nadolol. It is used as biomarkers. It is used in analytical chemistry as Molisch's reagent (1-naphthol dissolved in ethanol) for checking the presence of carbohydrates. It plays an essential role with sodium hypobromite to detect the presence of arginine in proteins, which is called as Sakaguchi test.
Application
1-Naphthol is a hydroxyl-aromatic compound. It has been used as a prooxidant to analyze its ability to induce hemolysis in a zebrafish G6PD (glucose-6-phosphate dehydrogenase) deficiency model.
Definition
ChEBI: 1-naphthol is a naphthol carrying a hydroxy group at position 1. It has a role as a genotoxin and a human xenobiotic metabolite.
Preparation
2-Isopropylnaphthalene can be used to synthesize 1-naphthol by oxidation via the hydroperoxide (Hock synthesis).
Historically 1-naphthol was produced via caustic fusion of naphthalene-1-sulfonic acid, but this was superseded by the I.G. Farbenindustrie process involving hydrolysis of 1-naphthylamine with aqueous 22 % sulfuric acid at 200°C under pressure in a lead-lined autoclave. To obtain a purer product, Union Carbide developed a process based on catalytic oxidation of tetralin to 1-tetralol and 1-tetralone followed by dehydrogenation. The two-stage catalytic process is claimed to give an overall yield of 72% 1-naphthol, with an overall efficiency of 97%.
General Description
1-Naphthol, a metabolite of carbaryl and naphthanlene. It is formed by spontaneous reaction from (1R, 2S)-Naphthalene epoxide followed to form 1, 4-Dihydroxynaphthalene.
Hazard
Toxic by ingestion and skin absorption.
Contact allergens
1-Naphthol can be used in dye manufacture and is classified as a hair dye. Combined with epichlorhydrin and NaOH to form alpha-naphthyl glycidyl ether, it caused sensitization in one of three workers in a chemical plant.
Safety Profile
Poison by ingestion and intraperitoneal routes. Moderately toxic by skin contact. An experimental teratogen. Experimental reproductive effects. A severe eye and skin irritant. Mutation data reported. Ingestion of large amounts can cause nephritis, vomiting, diarrhea, circulatory collapse, anemia, convulsions, and death. Can cause kidney irritation and injury to cornea and lens of the eye. Combustible when exposed to heat or flame. When heated to decomposition it emits acrid smoke and irritating fumes.
Synthesis
To a 10 mL pressure vessel, aryl halide (1.00 mmol), copper (I) oxide (0.05 mol), ligand (0.05 mol), 3 M sodium hydroxide (2 mL), and organic solvent (2 mL) were added. The reaction mixture was irradiated at 160 °C for 10 min with strong stirring. The reaction mixture was allowed to cool to room temperature. The reaction mixture was filtered through a plug of celite in a fritted filter funnel and washed with ethyl acetate. The product was extracted using 30 mL of ethyl acetate for three times. The organic extract is washed three times with 10 mL water and two times with 10 mL of brine. The combined organic phases were dried over anhydrous MgSO4 and the solvent was removed under reduced pressure to afford 1-naphthol.
Purification Methods
Sublime 1-naphthol, then crystallise it from aqueous MeOH (charcoal), aqueous 25% or 50% EtOH, *C6H6, cyclohexane, heptane, CCl4 or H2O. Dry it over P2O5 in vacuo. The 4-nitrobenzoate has m 143o (from EtOH). [Shizuka et al. J Am Chem Soc 107 7816 1985, Beilstein 8 H 596, 6 IV 4208.]
1-Naphthol Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Chemicea Pharmaceuticals Pvt Ltd | +91-918482849343 +91-8482849343 | Mumbai, India | 461 | 58 | Inquiry |
| Rsum Industries Pvt. Ltd. | +91-91062685255 +91-91062685255 | New Delhi, India | 29 | 58 | Inquiry |
| Arnish Laborates Pvt Ltd | +91 7208475595 | Maharashtra, India | 187 | 58 | Inquiry |
| SNECOFRi Pvt Ltd | +91-9032850129 +91-9032850129 | Telangana, India | 404 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| KARPSCHEM LABORATORIES | +91-7249203006 +91-7249203006 | Maharashtra, India | 786 | 58 | Inquiry |
| Varanous Labs Pvt Ltd | +91-7036248882 +91-7036248882 | Hyderabad, India | 1541 | 58 | Inquiry |
| Krishna Industries | +91-7922822554 +91-7922822554 | Gujarat, India | 235 | 58 | Inquiry |
| Vijaya Pharma And Life Science | +91-8939866271 +91-8939866271 | Tamil Nadu, India | 320 | 58 | Inquiry |
| Aspen Biopharma Labs Pvt Ltd | +91-9248058660 +91-9248058662 | Telangana, India | 234 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Chemicea Pharmaceuticals Pvt Ltd | 58 |
| Rsum Industries Pvt. Ltd. | 58 |
| Arnish Laborates Pvt Ltd | 58 |
| SNECOFRi Pvt Ltd | 58 |
| JSK Chemicals | 58 |
| KARPSCHEM LABORATORIES | 58 |
| Varanous Labs Pvt Ltd | 58 |
| Krishna Industries | 58 |
| Vijaya Pharma And Life Science | 58 |
| Aspen Biopharma Labs Pvt Ltd | 58 |
Related articles
- 1-Naphthol: Natural Occurrence and its Catabolism via Synergistic Regulation
- 1-Naphthol, a genotoxin and xenobiotic metabolite, occurs naturally, posing ecological, biochemical, and toxicological implica....
- May 17,2024
- 1-Naphthol: pharmacokinetics, activities and toxicity
- 1-Naphthol, a compound with antioxidant activity, exhibits first-order kinetics, but oral toxicity studies in mice showed acut....
- Jul 12,2023
- 1-Naphthol: Production and Uses
- The passage introduces the production and uses of 1-Naphthol.
- Nov 18,2022
90-15-3(1-Naphthol)Related Search:
1of4
chevron_right




