MERCURIC BENZOATE
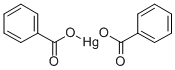
- CAS No.
- 583-15-3
- Chemical Name:
- MERCURIC BENZOATE
- Synonyms
- Mercury benzoate;mercurydibenzoate;MERCURIC BENZOATE;mercuric dibenzoate;beta-mercuribenzoate;MERCURY (II) BENZOATE;mercury(2+) dibenzoate;Quecksilber(II)-benzoat;benzoicacid,mercury(2+)salt;Dibenzoic acid mercury(II) salt
- CBNumber:
- CB0428907
- Molecular Formula:
- C14H10HgO4
- Molecular Weight:
- 442.82
- MOL File:
- 583-15-3.mol
- Modify Date:
- 2023/4/23 13:52:06
| Melting point | 166-167 °C(lit.) |
|---|---|
| form | solid |
| Water Solubility | 1.2 g/100mL H2O (15°C), 2.5g/100mL H2O (100°C) [CRC10] |
| CAS DataBase Reference | 583-15-3 |
| EPA Substance Registry System | Benzoic acid, mercury(2+) salt (583-15-3) |
SAFETY
Risk and Safety Statements
| Hazard Codes | T+,N |
|---|---|
| Risk Statements | 26/27/28-33-50/53 |
| Safety Statements | 13-28-36-45-60-61 |
| RIDADR | UN 1631 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | OV7060000 |
| HazardClass | 6.1(a) |
| PackingGroup | II |
MERCURIC BENZOATE Chemical Properties,Uses,Production
Chemical Properties
White crystals.Sensitive to light. Soluble in solutions of sodium chloride and ammonium benzoate; slightly soluble in alcohol and water.
Uses
Medicine (antisyphilitic).
General Description
A white crystalline odorless solid. Melting point 165°C. Sensitive to light. Highly toxic by inhalation and ingestion.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
MERCURIC BENZOATE is soluble in solutions of sodium chloride and ammonium benzoate [Hawley]. Reacts as a weak base.
Hazard
Toxic by ingestion, inhalation, and skin absorption; strong irritant.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
A poison. When heated to decomposition it emits toxic fumes of Hg. See also MERCURY COMPOUNDS.
MERCURIC BENZOATE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| City Chemicals Corporation | 800-248-2436 | United States | 6462 | 72 | Inquiry |
| MP Biomedicals, Inc. | 949 833 2500 | United States | 6566 | 81 | Inquiry |
| 3B Scientific Corporation | 847.281.9822 | United States | 6744 | 47 | Inquiry |
| City Chemical LLC | 1-203-932-2489 | United States | 6710 | 72 | Inquiry |
| CARBONE SCIENTIFIC CO.,LTD | +44(0)870 486 8629 | United Kingdom | 6682 | 30 | Inquiry |
| Leancare Ltd. | +33 962096793 | United Kingdom | 6460 | 42 | Inquiry |
| 3B Pharmachem (Wuhan) International Co.,Ltd. | 821-50328103-801 18930552037 | China | 15848 | 69 | Inquiry |
| Portail Substances Chimiques | 10 20 0000 | France | 6027 | 58 | Inquiry |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 15229059051 | China | 9971 | 58 | Inquiry |
| Henan Dobe Chemical Co. , Ltd. | 0370-6999054 17334723986 | China | 1336 | 58 | Inquiry |





