MERCURIC ACETATE
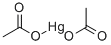
- CAS No.
- 1600-27-7
- Chemical Name:
- MERCURIC ACETATE
- Synonyms
- mercury(II) diacetate;MERCURY(II) ACETATE;MERCURY ACETATE;ai3-04458;MERCURICACID;mercuriacetate;mercurylacetate;MERCURIC ACERAE;mercurydiacetate;diacetoxymercury
- CBNumber:
- CB6300665
- Molecular Formula:
- C4H6HgO4
- Molecular Weight:
- 318.68
- MOL File:
- 1600-27-7.mol
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 179-182 °C(lit.) |
|---|---|
| bulk density | 1000kg/m3 |
| Density | 3,29 g/cm3 |
| vapor density | 11 (vs air) |
| storage temp. | Store at RT. |
| solubility | Water (Slightly) |
| form | Solid |
| Specific Gravity | 3.27 |
| color | White to almost white |
| Odor | Slight acetic acid odor |
| Water Solubility | Soluble in water. |
| Sensitive | Light Sensitive |
| Hydrolytic Sensitivity | 0: forms stable aqueous solutions |
| Merck | 14,5873 |
| BRN | 3563831 |
| Stability | Stable. Incompatible with strong oxidizing agents, strong bases. May decompose upon exposure to light. |
| CAS DataBase Reference | 1600-27-7(CAS DataBase Reference) |
| EPA Substance Registry System | Mercuric acetate (1600-27-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310+H330-H373-H410 | |||||||||
| Precautionary statements | P262-P273-P280-P302+P352+P310-P304+P340+P310-P314 | |||||||||
| Hazard Codes | T+,N | |||||||||
| Risk Statements | 26/27/28-33-50/53 | |||||||||
| Safety Statements | 13-28-36-45-60-61 | |||||||||
| RIDADR | UN 1629 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | AI8575000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28521000 | |||||||||
| Hazardous Substances Data | 1600-27-7(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
MERCURIC ACETATE price More Price(21)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 83352 | Mercury(II) acetate puriss. p.a., ACS reagent, ≥99.0% (precipitation titration) | 1600-27-7 | 50G | ₹6332.63 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 83352 | Mercury(II) acetate puriss. p.a., ACS reagent, ≥99.0% (precipitation titration) | 1600-27-7 | 250G | ₹21141.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 176109 | Mercury(II) acetate ACS reagent, ≥98.0% | 1600-27-7 | 5G | ₹2933.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 176109 | Mercury(II) acetate ACS reagent, ≥98.0% | 1600-27-7 | 100G | ₹7285.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 176109 | Mercury(II) acetate ACS reagent, ≥98.0% | 1600-27-7 | 500G | ₹29119.25 | 2022-06-14 | Buy |
MERCURIC ACETATE Chemical Properties,Uses,Production
Description
Mercury (II) acetate is the chemical compound with the formula Hg(O2CCH3)2. Commonly abbreviated Hg (OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors.
Chemical Properties
Mercuric acetate, Hg(C2H3O2)2 , is a toxic, light-sensitive white powder, soluble in water,alcohol,and acetic acid. On exposure to heat, mercuric acetate produces toxic fumes of mercury/mercuric oxide. Mercuric acetate is incompatible with chromic acid, chromic anhydride, nitric acid, perchloric acid, permanganates, sodium peroxide, potassium hydroxide, hydrogen peroxides, acid anhydrides, and strong oxidising agents.
Physical properties
Mercury(II) acetate is a crystalline solid consisting of isolated Hg(OAc)2 molecules with Hg-O distances of 2.07 ?. Three long, weak intermolecular Hg···O bonds of about 2.75 ? are also present,resulting in a slightly distorted square pyramidal coordination geometry at Hg.
Uses
Mercuric acetate is used as an oxidizing agent in organic synthesis. It is used in oxymercuration of double bonds. Mercuric acetate is used in non-aqueous titration. It is employed in the manufacture of phenyl mercury compounds which have pharmaceutical applications. It removes the acetamidomethyl protecting group from protected thiol, and converts thiocarbonate esters into dithiocarbonates. It promotes the addition of hydroxide and alkoxide across carbon-carbon double bonds.
Reactions
Arenes undergo "mercuration" upon treatment with Hg(OAc)2. The one acetate group that remains on mercury can be displaced by chloride :
C6H5OH + Hg(OAc)2 → C6H4(OH)-2-HgOAc + HOAc
C6H4(OH)-2-HgOAc + NaCl → C6H4(OH)-2-HgCl + NaOAc
The Hg2+ center binds to alkenes, inducing the addition of hydroxide and alkoxide. For example, treatment of methylacrylate with mercuric acetate in methanol gives an α - mercuri ester :
Hg(OAc)2 + CH2 = CHCO2CH3 + CH3OH → CH3OCH2CH(HgOAc)CO2CH3+ HOAc
Mercury(II) has a high affinity for sulfur ligands. Hg (OAc)2 can be used as a reagent to remove the acetamidomethyl protecting group, which is used to "protect" thiol groups in organic synthesis. Similarly Hg(OAc)2 is a standard reagent to convert thiocarbonate esters into dithiocarbonates:
(RS)2C=S + H2O + Hg(OAc)2 → (RS)2C=O + HgS + 2 HOAc
Mercury (II) acetate is used for oxymercuration reactions.
General Description
White crystalline solid with an odor of vinegar. Sensitive to light. Density 3.25 g / cm3. Toxic by inhalation (dust, etc.) and by ingestion.
Air & Water Reactions
Water soluble. Decomposed by water to form a yellow insoluble product.
Reactivity Profile
MERCURIC ACETATE is incompatible with acetylene, ammonia, chlorine dioxide, azides, calcium (amalgam formation), sodium carbide, lithium, rubidium, and copper .
Hazard
Toxic by ingestion, inhalation, and skin absorption; strong irritant.
Health Hazard
MERCURIC ACETATE may cause death by hypovolemic shock or kidney failure. Chronic exposure may lead to kidney failure.
Fire Hazard
When heated to decomposition, MERCURIC ACETATE emits toxic fumes of mercury. Avoid light.
Safety Profile
Poison by ingestion, intravenous, intraperitoneal, and subcutaneous routes. Moderately toxic by skin contact. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of Hg. See also MERCURY COMPOUNDS.
Potential Exposure
Mercuric acetate is used chiefly for mercuration of organic compounds; for the absorption ofethylene; as a chemical intermediate for phenylmercuric acetate; a mildewcide; and other organomercury compounds. It is used as a catalyst in organic synthesis; and in the manufacture of pharmaceuticals.
Shipping
UN1629 Mercury acetate, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Recrystallise it from glacial acetic acid. POISONOUS. [Beilstein 2 IV 114.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Light and heat can cause decomposition.
MERCURIC ACETATE Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
chevron_right1600-27-7(MERCURIC ACETATE)Related Search:
1of4
chevron_right




