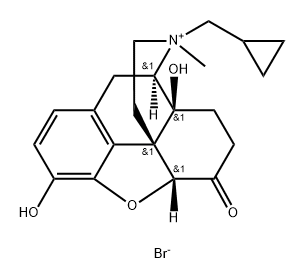
Naltrexone methylbromide
- CAS No.
- 73232-52-7
- Chemical Name:
- Naltrexone methylbromide
- Synonyms
- MNTX;Moa-728;Relistor;Unii-rfo6il3D3m;Adamantane Impurity 52;Naltrexone methobromide;Methylnaltrexone bromide;Naltrexone methylbromide;Methyhaaltrexone broMide;N-Methyl Naltrexone BroMide
- CBNumber:
- CB11105859
- Molecular Formula:
- C21H26BrNO4
- Molecular Weight:
- 436.35
- MOL File:
- 73232-52-7.mol
- Modify Date:
- 2024/3/19 15:37:50
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H400 | |||||||||
| Precautionary statements | P273-P301+P310 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 25-50 | |||||||||
| Safety Statements | 45-61 | |||||||||
| RIDADR | UN 2811 6.1 / PGIII | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | QD0188550 | |||||||||
| HS Code | 2934990002 | |||||||||
| NFPA 704 |
|
Naltrexone methylbromide Chemical Properties,Uses,Production
Description
The widespread efficacy of opioids in treating patients with moderate to
severe acute and chronic pain is often accompanied by untoward side
effects. In particular, opioid-induced bowel dysfunction is one of the
more common and debilitating consequences afflicting up to 50% of
patients. To counteract the peripherally-mediated adverse effects, opioid
antagonists such as naloxone, naltrexone, and nalmephene are sometimes prescribed. The latest market entry exploits a strategic
modification of naltrexone to lower its lipid solubility and increase its
polarity: quaternization of the amine of naltrexone by methylation
(methyl bromide) prevents crossing of the blood–brain barrier thereby
creating an effective peripheral antagonist. Despite a loss of potency
upon methylation, methylnaltrexone antagonizes opioid binding at
m-opioid receptors with an IC50 of 70 nM. Its affinity for k-opioid receptors
is approximately eightfold less (IC50= 575 nM) with no significant binding
to d-opioid, orphanin, or non-opioid receptors. Methylnaltrexone bromide
has been approved for the treatment of opioid-induced constipation in
patients with advanced illness receiving palliative care.Regarding metabolism, methylnaltrexone bromide is eliminated primarily as intact drug (85% based on administered radioactivity) by slightly more renal than hepatic clearance.
The most common adverse events were abdominal pain and flatulence followed by nausea, dizziness, and diarrhea.
Chemical Properties
Pale Pink Solid
Uses
A metabolite of Naltrexone (N285750). Methylnaltrexone (MNTX), a selective μ-opioid receptor antagonist, functions as a peripherally acting receptor antagonist in tissues of the gastrointestinal tract.
General Description
Methylnaltrexone does not cross blood brain barrier and does not affect the opioid effects in the brain, such as analgesia. It is used to treat opioid-induced constipation (OIC).
Naltrexone methylbromide Preparation Products And Raw materials
Raw materials
Preparation Products
Naltrexone methylbromide Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Rusan Pharma Ltd | +91-2228687035 +91-9690022465 | Maharashtra, India | 14 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21667 | 55 | Inquiry |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | China | 10311 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49392 | 58 | Inquiry |
| SIMAGCHEM CORP | +86-13806087780 | China | 17367 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30250 | 58 | Inquiry |
| Win-Win chemical CO., Limited | +86-0086-577-64498589 +86-15355981851 | China | 14351 | 58 | Inquiry |





