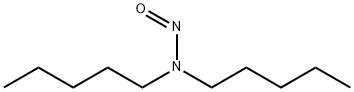
N,N-Diamylnitrosamine
- CAS No.
- 13256-06-9
- Chemical Name:
- N,N-Diamylnitrosamine
- Synonyms
- NSC 7360;NSC 73601;Diamylnitrosamin;Diamylnitrosamine;NITROSODIAMYLAMINE;Dipentylnitrosamine;Di-N-amylnitrosamine;Dipentylnitrosoamine;N-Nitrosodiamylamine;N,N-Diamylnitrosamine
- CBNumber:
- CB11176514
- Molecular Formula:
- C10H22N2O
- Molecular Weight:
- 186.29
- MOL File:
- 13256-06-9.mol
- Modify Date:
- 2023/4/23 13:52:06
| Boiling point | 320.8°C (rough estimate) |
|---|---|
| Density | 0.9698 (rough estimate) |
| refractive index | 1.4710 (estimate) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | Oil |
| pka | -3.15±0.70(Predicted) |
| color | Clear Colourless to Pale Yellow |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 13256-06-9 |
| EPA Substance Registry System | N,N,-Diamylnitrosamine (13256-06-9) |
N,N-Diamylnitrosamine Chemical Properties,Uses,Production
Chemical Properties
colourless to light yellow liquid
Uses
N,N-Diamylnitrosamine (cas# 13256-06-9) is a compound useful in organic synthesis.
General Description
Clear light yellow liquid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
A nitrated amine. Amines are combustible. The combustion of amines yields noxious NOx. REACTIVITY - Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Aromatic nitro compounds range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups.
N,N-Diamylnitrosamine Preparation Products And Raw materials
N,N-Diamylnitrosamine Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLP Pharma Standards | +91 9866074638 | Hyderabad, India | 1644 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Aikon International Limited | 025-66113011 18112977050 | China | 15495 | 58 | Inquiry |
| Nanjing bojida Pharmaceutical Technology Co., Ltd | 18762407961 | China | 657 | 58 | Inquiry |
| guoyungurui | 18162595016 | China | 10338 | 58 | Inquiry |
| Nanjing XiZe Biotechnology CO., Ltd. | 025-66023220 0086-15250997978 | China | 7786 | 55 | Inquiry |
| HONEST JOY HOLDINGS LIMITED | +86-755-26404303 | United States | 6702 | 54 | Inquiry |
| Medical Isotopes | 1-(603) 635-1722 | United States | 6185 | 68 | Inquiry |
| Toronto Research Chemicals | +1 (416) 665-9696 | Canada | 6088 | 71 | Inquiry |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | China | 96815 | 76 | Inquiry |





