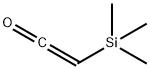
Trimethylsilylketene
- CAS No.
- 4071-85-6
- Chemical Name:
- Trimethylsilylketene
- Synonyms
- Trimethylsilylketene;(Trimethylsilyl)ketene;(trimethylsilyl)-ethenon;2-trimethylsilylethenone;(TRIMETHYLSILYL)KETENE 97;(TRIMETHYLSILYL)KETENE 97%;Ethenone, 2-(trimethylsilyl)-;2-(Trimethylsilyl)ethene-1-one
- CBNumber:
- CB1500224
- Molecular Formula:
- C5H10OSi
- Molecular Weight:
- 114.22
- MOL File:
- 4071-85-6.mol
- Modify Date:
- 2023/5/4 15:12:37
| Boiling point | 80-82 °C(lit.) |
|---|---|
| Density | 0.813 g/mL at 25 °C(lit.) |
| refractive index |
n |
| Flash point | 3 °F |
| solubility | Sol CH2Cl2, CHCl3, CCl4, THF, diethyl ether, and most standard organic solvents; reacts with alcoholic and amine solvents. |
| EPA Substance Registry System | Ethenone, (trimethylsilyl)- (4071-85-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS02 |
|---|---|
| Signal word | Danger |
| Hazard statements | H225 |
| Precautionary statements | P210-P233-P240-P241-P242-P243-P280-P303+P361+P353-P370+P378-P403+P235-P501 |
| Hazard Codes | F |
| Risk Statements | 11 |
| Safety Statements | 7/9-16-29-33 |
| RIDADR | UN 1993 3/PG 2 |
| WGK Germany | 3 |
Trimethylsilylketene Chemical Properties,Uses,Production
Physical properties
bp 81–82 °C; d 0.80 g cm?3.
Uses
Trimethylsilylketene is a reactive acylating agent for amines and alcohols; building block for synthesis of coumarins; synthesis of α-silyl ketones via the addition of organocerium reagents; treatment with stabilized ylides forms trimethylsilyl-substituted allenes;a cycloaddition with aldehydes affords β-lactones; forms small rings with diazomethane; treatment with n-BuLi forms a ketene enolate. It participates in the reactions of Trimethylsilylacetylation of Alcohols and Amines, Synthesis of Coumarins via Cyclization–Elimination, One-pot Formation of α-Silyl Ketones, Preparation of Trimethylsilyl-Substituted Allenes, Preparation of β-Lactones, Reaction with Diazomethane to Form Silylated Cyclopropanes and Cyclobutanones, Synthesis of Heterocycles, Formation of the Ketene Enolate, and other uses.
Preparation
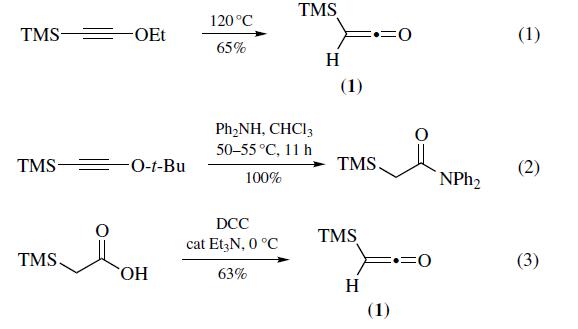
Most often prepared (eq 1) by pyrolysis of ethoxy(trimethylsilyl)acetylene at 120??C (100 mmol scale, 65% yield).Recently, pyrolysis of t-butoxy(trimethylsilyl) acetylene has been shown to be a convenient alternative for the preparation of trimethylsilylketene (1). Thermal decomposition of t-butoxy(trimethylsilyl)acetylene causes elimination of 2-methylpropene slowly at temperatures as low as 50??C and instantaneously at 100¨C110??C (30 mmol scale, 63% yield). The main advantage of this method is that it is possible to generate trimethylsilylketene in the presence of nucleophiles, leading to in situ trimethylsilylacetylation (eq 2). Increased shielding of the triple bond prevents problems such as polymerization and nucleophilic attack that occur when the ketene is generated in situ from (trimethylsilyl)ethoxyacetylene. Trimethylsilylketene can also be prepared (eq 3) via the dehydration of commercially available trimethylsilylacetic acid with 1,3- dicyclohexylcarbodiimide (DCC) in the presence of a catalytic amount of triethylamine (100 mmol scale, 63%). Other typical methods used for ketene generation such as dehydrohalogenation of the acyl chloride and pyrolysis of the anhydride have been applied to the preparation of (1); however, both methods afford low yields.
Trimethylsilylketene Preparation Products And Raw materials
Raw materials
Preparation Products
Trimethylsilylketene Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503259 +86-19930503259 | China | 18439 | 58 | Inquiry |
| ABCR GmbH & CO. KG | 49 721 95061 0 | Germany | 6846 | 75 | Inquiry |
| SIGMA-RBI | 800 736 3690 (Orders) | Switzerland | 6913 | 91 | Inquiry |
| Reagent World | +1 (909) 947-7779 | United States | 1637 | 50 | Inquiry |
| Alfa Chemistry | +1 (201) 478-8534 | United States | 6824 | 0 | Inquiry |
| Riedel-de Haen AG | 800 558-9160 | United States | 6825 | 87 | Inquiry |
| Supplier | Advantage |
|---|---|
| CONIER CHEM AND PHARMA LIMITED | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | 58 |
| ABCR GmbH & CO. KG | 75 |
| SIGMA-RBI | 91 |
| Reagent World | 50 |
| Alfa Chemistry | 0 |
| Riedel-de Haen AG | 87 |





