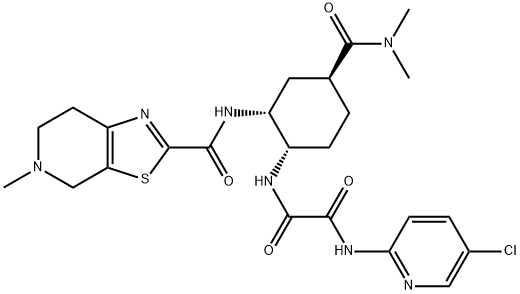
edoxaban
- CAS No.
- 480449-70-5
- Chemical Name:
- edoxaban
- Synonyms
- Edoxaban base;EthanediaMide, N1-(5-chloro-2-pyridinyl)-N2-[(1S,2R,4S)-4-[(diMethylaMino)carbonyl]-2-[[(4,5,6,7-tetrahydro-5-Methylthiazolo[5,4-c]pyridin-2-yl)carbonyl]aMino]cyclohexyl]-;DU-176;edoxaban;Edoxaban(DU-176);edoxaban USP/EP/BP;edoxaban480449-70-5;Edoxaban/Ethanediamide;Edoxaban (See C3D-1344);Tropicamide Impurity 34
- CBNumber:
- CB21518508
- Molecular Formula:
- C24H30ClN7O4S
- Molecular Weight:
- 548.06
- MOL File:
- 480449-70-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/21 22:41:43
| Melting point | >213°C (dec.) |
|---|---|
| Density | 1.43 |
| storage temp. | Hygroscopic, Refrigerator, under inert atmosphere |
| solubility | Chloroform (Slightly), DMSO (Slightly, Heated), Methanol (Slightly, Heated) |
| pka | 9.46±0.70(Predicted) |
| form | Solid |
| color | White to Off-White |
| InChIKey | HGVDHZBSSITLCT-JLJPHGGASA-N |
| SMILES | C(NC1=NC=C(Cl)C=C1)(=O)C(N[C@H]1CC[C@H](C(N(C)C)=O)C[C@H]1NC(C1=NC2CCN(C)CC=2S1)=O)=O |
| LogP | -1.455 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H373 |
| Precautionary statements | P260-P314-P501 |
edoxaban Chemical Properties,Uses,Production
Uses
Edoxaban is an anticoagulant drug which acts as a direct factor Xa inhibitor.
Indications
Edoxaban is a direct oral anticoagulant (DOAC). It exerts its effects by inhibiting factor Xa (FXa). Edoxaban was FDA approved in early 2015 to treat deep venous thrombosis, pulmonary embolism and decrease the risk of hypercoagulability related illness; stroke, and systemic embolism (SE) in subjects with nonvalvular atrial fibrillation (NVAF).When compared to the popularly used anticoagulant warfarin, edoxaban entails more limited monitoring and possesses a reduced risk for significant bleeding and fewer interactions with other agents.Edoxaban is the most current direct oral anticoagulant (DOAC) and does not associate with the CYP-450 system.
Various clinical trials and studies (ENGAGE AF-TIMI and Hokusai-VTE) expressed edoxaban's effectiveness compared to the conventional vitamin K antagonist warfarin. It was demonstrated to be non-inferior in preventing blood clots when compared to warfarin. Edoxaban is the second FDA-approved anticoagulant agent with once-daily administration.Contrary to the other direct oral anticoagulants, apixaban and rivaroxaban, edoxaban has not yet received approval for secondary and postoperative prophylaxis for venous thromboembolism (VTE).
FDA-Approved Indications:
Deep venous thrombosis
Pulmonary embolism
Nonvalvular atrial fibrillation (NVAF)
https://www.ncbi.nlm.nih.gov/
Definition
ChEBI: A monocarboxylic acid amide that is used (as its tosylate monohydrate) for the treatment of deep vein thrombosis and pulmonary embolism.
Pharmacology
Similar to rivaroxaban and apixaban,edoxaban is an orally active, small-molecule (548 Da), reversible factor Xa inhibitor.As with the other direct oral factor Xa inhibitors,edoxaban exhibits a 10,000-fold greater selectivity for factor Xa compared with other serine proteins such as factor VlIa, t-PA, plasmin,or trypsin.Administered as edoxaban tosylate,the compound competitively inhibits free factor Xa directly without the need for antithrombin and factor Xa incorporated in the prothrombinase complex. The concentration-dependent inhibition of factor Xa leads to reduced thrombin generation and thrombin-induced platelet aggregation. Edoxaban inhibited factor Xa with Ki values of 0.561 nM for free factor Xa and 2.98 nM for prothrombinase.128 Edoxaban exhibits linear pharmacokinetics and produces concentration-dependent increases in the PT,INR, and aPTT.However,changes in these laboratory assays with edoxaban tend to be unpredictable and highly variable, reducing their use as a monitoring tool in clinical practice.
Pharmacokinetics and Pharmacodynamics of Edoxaban, a Non-Vitamin K Antagonist Oral Anticoagulant that Inhibits Clotting Factor Xa
Laboratory measurement of the anticoagulant activity of edoxaban: a systematic review
Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans
Drug interactions
The majority of edoxaban pharmacokinetic drug interactions result from inhibition or induction of the P-gp efflux transporter,which is responsible for intestinal transport.Edoxaban taken with quinidine demonstrates an increase in edoxaban Cmax of 85% and AUS of 77%.Coadministration with dronedarone resulted in a Cmax and AUC increase of 46% and 85%,respectively.This drug interaction also increased the 24-h edoxaban concentration by 158%.Additionally, verapamil increased the edoxaban Cmax by 53%, the AUC by 53%, and the 24-h edoxaban concentration by 29%.146 As per the phase 3 clinical trial, patients receiving quinidine, dronedarone,or verapamil should receive the reduced dose of 30 mg daily instead of 60 mg daily.It should be noted that patients receiving azole antifungal agents, such as ketoconazole, or protease inhibitors were excluded from the phase 3 trial because of concerns about increased edoxaban exposure.Conversely, the use of rifampin, a P-gp inducer, resulted in a significant 34% reduction in the edoxaban AUC.Therefore, the combination of rifampin and edoxaban should be avoided.
edoxaban Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Saptagir Laboratories PVT.LTD. | +91-9059978049 +91-9059978049 | Telangana, India | 31 | 58 | Inquiry |
| Dr. Reddy's Laboratories Ltd | +91-4049002900 +91-4049002900 | Hyderabad, India | 165 | 58 | Inquiry |
| Hikal Ltd. | +91-9538444622 +91-8067356100 | Maharashtra, India | 31 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Ami Lifesciences Pvt Ltd | +91-9426998100 +91-9426998100 | Gujarat, India | 62 | 58 | Inquiry |
| Vijaya Pharma And Life Science | +91-8939866271 +91-8939866271 | Tamil Nadu, India | 320 | 58 | Inquiry |
| Anlon Healthcare Pvt Ltd | +91-2812562538 +91-7069690081 | Gujarat, India | 54 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Libertas | 08069033735Ext 211 | Gujarat, India | 29 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
Related articles
- Introduction of Edoxaban
- Edoxaban is an orally active inhibitor of coagulation factor Xa (activated factor X) with anticoagulant activity. Edoxaban is ....
- Dec 7,2021
480449-70-5(edoxaban)Related Search:
1of4
chevron_right




