ChemicalBook > Product Catalog >Analytical Chemistry >Standard >Pharmaceutical Impurity Reference Standards >Miconazole IMpurity I
Miconazole IMpurity I
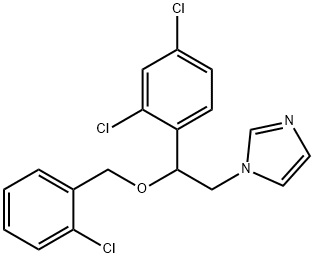
- CAS No.
- 47363-37-1
- Chemical Name:
- Miconazole IMpurity I
- Synonyms
- Miconazole EP IMP I;Miconazole IMpurity I;Miconazole Impurity I(EP);Miconazole EP Impurity I HCl;Miconazole Nitrate EP Impurity I;Miconazole Impurity 9(Miconazole EP Impurity I);1-[2-(2-chloro-benzyloxy)-2-(2,4-dichloro-phenyl)-ethyl]-1H-imidazole;1H-Imidazole, 1-[2-[(2-chlorophenyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-;1-[(2RS)-2-[(2-Chlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole;1-(2-((2-Chlorobenzyl)oxy)-2-(2,4-dichlorophenyl)ethyl)-1H-imidazole (Miconazole Impurity)
- CBNumber:
- CB22674890
- Molecular Formula:
- C18H15Cl3N2O
- Molecular Weight:
- 381.68
- MOL File:
- 47363-37-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/4/23 13:52:06
Miconazole IMpurity I Preparation Products And Raw materials
Raw materials
Preparation Products
Global( 40)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLP Pharma Standards | +91 9866074638 | Hyderabad, India | 1644 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30250 | 58 | Inquiry |
| China National Standard Pharmaceutical Corporation Limited | +8615391658522 | China | 11927 | 58 | Inquiry |
| Guangzhou PI PI Biotech Inc | 020-81716320 +86-13602409664 | China | 3635 | 55 | Inquiry |
| Guangzhou Dreampharm Biotechnology Co., Ltd. | 020-36607679 17825480238 | China | 9919 | 12 | Inquiry |
| Shanghai Haohong Scientific Co., Ltd. | 400-400-8210725 | China | 39953 | 58 | Inquiry |
| Nanjing XiZe Biotechnology CO., Ltd. | 025-66023220 0086-15250997978 | China | 7851 | 55 | Inquiry |
| Shanghai YuZn Pharm. Technology Co.,Ltd. | 15921286451; 15921286451 | China | 5096 | 58 | Inquiry |
47363-37-1(Miconazole IMpurity I)Related Search:
Miconazole IMpurity H
2,4-Dichlorobenzyl alcohol
Econazole
Miconazole
alpha-(2,4-Dichlorophenyl)-1H-imidazole-1-ethanol
Miconazole EP IMpurity C
Milbemycin EP Impurity E
Milrinone Impurity 14
Milrinone Impurity 17
5-Decyano 5-(Ethyl Formate) Milrinone
chevron_left
1of4
chevron_right
Miconazole IMpurity I
Miconazole EP IMP I
Miconazole Impurity I(EP)
Miconazole EP Impurity I HCl
Miconazole Impurity 9(Miconazole EP Impurity I)
Miconazole Nitrate EP Impurity I
1-[2-(2-chloro-benzyloxy)-2-(2,4-dichloro-phenyl)-ethyl]-1H-imidazole
1H-Imidazole, 1-[2-[(2-chlorophenyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-
Miconazole EP impurity IQ: What is
Miconazole EP impurity I Q: What is the CAS Number of
Miconazole EP impurity I Q: What is the storage condition of
Miconazole EP impurity I Q: What are the applications of
Miconazole EP impurity I
1-[(2RS)-2-[(2-Chlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole
1-(2-((2-Chlorobenzyl)oxy)-2-(2,4-dichlorophenyl)ethyl)-1H-imidazole (Miconazole Impurity)
47363-37-1





