GLYCIDALDEHYDE
- CAS No.
- 765-34-4
- Chemical Name:
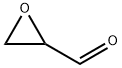
- GLYCIDALDEHYDE
- Synonyms
- glycidal;formyloxiran;GLYCIDALDEHYDE;glycinaldehyde;glycidylaldehyde;epihydrinaldehyde;2,3-epoxypropanal;epihydrinealdehyde;(R)-GLYCIDALDEHYDE;rcrawastenumberu126
- CBNumber:
- CB2474630
- Molecular Formula:
- C3H4O2
- Molecular Weight:
- 72.06
- MOL File:
- 765-34-4.mol
- Modify Date:
- 2023/5/15 10:43:55
| Melting point | -62°C |
|---|---|
| Boiling point | 86.43°C (rough estimate) |
| Density | 1.4170 |
| refractive index | 1.6020 (estimate) |
| solubility | soluble in Chloroform, Ethyl Acetate |
| form | Oil |
| color | Cleat Colorless |
| Exposure limits | No exposure limit is set. It is recommended that human exposure to glycidaldehyde in the work environment should not exceed 1 ppm (3 mg/m3 ) concentration in air. |
| IARC | 2B (Vol. 11, Sup 7, 71) 1999 |
| EPA Substance Registry System | Glycidylaldehyde (765-34-4) |
GLYCIDALDEHYDE Chemical Properties,Uses,Production
Chemical Properties
Glycidaldehyde is a mobile, colorless liquid with a pungent odor.There is a pronounced aldehyde-like odor at low levels. Voluntary exposure to serious lung-irritating levels is unlikely.
Uses
Glycidaldehyde is prepared from the hydrogen peroxide epoxidation of acrolein. It is suggested as a bifunctional chemical intermediate and as a cross-linking agent for textile treatment, leather tanning, and protein insolubilization.
General Description
A colorless liquid. Slightly denser than water and insoluble in water. Flash point near 100°F. May irritate skin and eyes. Toxic by ingestion and inhalation. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
GLYCIDALDEHYDE is an epoxide and an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. Epoxides are highly reactive. They polymerize in the presence of catalysts or when heated. These polymerization reactions can be violent. Compounds in this group react with acids, bases, and oxidizing and reducing agents. They react, possibly violently with water in the presence of acid and other catalysts.
Health Hazard
Glycidaldehyde is a severe irritant, moder-ately toxic, and a carcinogenic compound.Exposure to 1 ppm for 5 minutes resultedin moderate eye irritation in humans. It pro-duced severe skin irritation with slow heal-ing, causing pigmentation of affected areas(Rose 1983).
The symptoms of its toxicity in humansare central nervous system depression, excite-ment, and effects on olfactory sense organs.Such ill effects may be observed on exposureto concentrations exceeding 5 ppm.
An intravenous administration of glyci-daldehyde at 20 mg/kg in rabbits causedmiosis, lacrimation, and respiratory depres-sion followed by death. In rats, 50 mg/kg,given orally, was fatal.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion and poison hazard indoors, outdoors or in sewers. May polymerize explosively when heated or involved in a fire. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Confirmed carcinogen with experimental carcinogenic,neoplastigenic, and tumorigenic data. Poison by ingestion, skin contact, intraperitoneal, and intravenous routes. Moderately toxic by inhalation. Human systemic effects by inhalation: changes in central nervous system electrical activity, olfactory changes, and excitement. Mutation data reported. A human eye irritant. Powerful skin sensitizer and mucous membrane irritant. Flammable when exposed to heat, flame, or oxidizing materials. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALDEHYDES.
Potential Exposure
Glycidyldehyde is and epoxide used to synthesize other chemicals. It has been used in the fin ishing of wool and the tanning of leather and surgical sutures in the U.K. It has been tested as a disinfectant.
Shipping
UN2622 Glycidaldehyde, Hazard Class: 3; Labels: 3-Flammable liquid, 6.1-Poisonous materials. The addition of antioxidant stabilizers to shipments of alde hydes may retard autoxidation.
Incompatibilities
Glycidaldehyde may undergo violent polymerization when subjected to heat, strong sunlight, or contamination. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. When heated or in contact with catalysts, epoxides may cause violent polymer ization. Epoxides are incompatible with reducing agents and oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materi als, strong bases, strong acids, oxoacids, and epoxides. May react, possibly violently, with water in the presence of acid and other catalysts. Reacts with alcohols, amines, and other active hydrogen compounds. Slowly hydrolyzes in water.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations govern ing storage, transportation, treatment, and waste disposal.
GLYCIDALDEHYDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| Portail Substances Chimiques | 10 20 0000 | France | 6027 | 58 | Inquiry |
| United States Biological | 800.520.3011 or 781.639.5092 | United States | 6256 | 80 | Inquiry |
| Sarchem Laboratories, Inc. | (732) 938-3777 | United States | 738 | 58 | Inquiry |
| City Chemicals Corporation | 800-248-2436 | United States | 6462 | 72 | Inquiry |
| Service Chemical Inc. | 888-895-6920 | Germany | 6373 | 71 | Inquiry |
| HONEST JOY HOLDINGS LIMITED | +86-755-26404303 | United States | 6702 | 54 | Inquiry |
| City Chemical LLC | 1-203-932-2489 | United States | 6710 | 72 | Inquiry |
| Leancare Ltd. | +33 962096793 | United Kingdom | 6460 | 42 | Inquiry |
| Supplier | Advantage |
|---|---|
| CLEARSYNTH LABS LTD. | 58 |
| Career Henan Chemica Co | 58 |
| Portail Substances Chimiques | 58 |
| United States Biological | 80 |
| Sarchem Laboratories, Inc. | 58 |
| City Chemicals Corporation | 72 |
| Service Chemical Inc. | 71 |
| HONEST JOY HOLDINGS LIMITED | 54 |
| City Chemical LLC | 72 |
| Leancare Ltd. | 42 |
765-34-4(GLYCIDALDEHYDE)Related Search:
1of4
chevron_right




