ChemicalBook > Product Catalog >API >Synthetic Anti-infective Drugs >Antifungal Drugs >CHRYSOMYCIN A
CHRYSOMYCIN A
- CAS No.
- 82196-88-1
- Chemical Name:
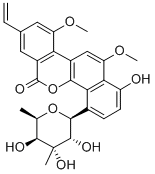
- CHRYSOMYCIN A
- Synonyms
- NSC 613946;A,ChrysoMycin;Virenomycin V;Chrysomycin V;(+)-Virenomycin V;CHRYSOMYCIN A USP/EP/BP;ChrysoMycin V, VirenoMycin V, Albacarcin V;8-ethenyl-1-hydroxy-10,12-dimethoxy-4-(3,4,5-trihydroxy-4,6-dimethyloxan-2-yl)naphtho[1,2-c]isochromen-6-one;6H-Benzo[d]naphtho[1,2-b]pyran-6-one, 4-(6-deoxy-3-C-methyl-β-gulopyranosyl)-8-ethenyl-1-hydroxy-10,12-dimethoxy-;4-(6-Deoxy-3-C-methyl-beta-gulopyranosyl)-8-ethenyl-1-hydroxy-10,12-dimethoxy-6H-benzo[d]naphtho[1,2-b]pyran-6-one
- CBNumber:
- CB2497985
- Molecular Formula:
- C28H28O9
- Molecular Weight:
- 508.52
- MOL File:
- 82196-88-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/6/30 15:45:59
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H312-H319-H351 | |||||||||
| Precautionary statements | P201-P202-P264-P270-P280-P301+P312-P330-P302+P352-P305+P351+P338-P308+P313-P321-P362+P364-P337+P313-P405-P501 | |||||||||
| WGK Germany | 3 | |||||||||
| NFPA 704 |
|
CHRYSOMYCIN A Chemical Properties,Uses,Production
Uses
Chrysomycin A is the major analogue in a complex of C-glycoside antitumour actives isolated from Streptomyces. Chrysomycin A, with a vinyl group in the 8-position, is the most potent analogue of the complex, and is thought to act as an inhibitor of the catalytic activity of human topoisomerase II. Chrysomyin A has a potent antibacterial, antifungal, antiviral and antitumour profile. More recent research on related metabolites, the gilvocarcins, suggests that chrysomycins may act as photoactivated, crosslinkers of DNA to histones.
CHRYSOMYCIN A Preparation Products And Raw materials
Raw materials
Preparation Products
Global( 36)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 24607 | 58 | Inquiry |
| BOC Sciences | 16314854226; +16314854226 | United States | 19743 | 58 | Inquiry |
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +86-19164747840 +86-13119157289 | China | 2971 | 58 | Inquiry |
| TargetMol Chemicals Inc. | 15002134094 | China | 28091 | 58 | Inquiry |
| Taizhou Nanfeng Pharmaceutical Research Institute | 18616377689 | China | 20566 | 58 | Inquiry |
| SHANG HAI WEI LING BIOTECHNOLOGY CO.,LTD | 13917490980 | China | 338 | 58 | Inquiry |
| Changzhou Bojia Biomedical Technology Co., Ltd. | 2122619822 | China | 18488 | 58 | Inquiry |
| Cayman Chemical Company | (800) 364-9897 | United States | 6618 | 81 | Inquiry |
| Shanghai EFE Biological Technology Co., Ltd. | 021-65675885 18964387627 | China | 9707 | 58 | Inquiry |
82196-88-1(CHRYSOMYCIN A)Related Search:
6H-Benzo[d]naphtho[1,2-b]pyran-6-one,4-(6-deoxy-3-C-methyl-b-gulopyranosyl)-8-ethenyl-1-hydroxy-10,12-dimethoxy-(9CI)
(+)-4-(6-Deoxy-3-C-methyl-β-D-gulo-hexopyranosyl)-8-ethenyl-1-hydroxy-10,12-dimethoxy-6H-benzo[d]naphtho[1,2-b]pyran-6-one
(+)-Virenomycin V
Chrysomycin V
Virenomycin V
4-(6-Deoxy-3-C-methyl-beta-gulopyranosyl)-8-ethenyl-1-hydroxy-10,12-dimethoxy-6H-benzo[d]naphtho[1,2-b]pyran-6-one
NSC 613946
A,ChrysoMycin
ChrysoMycin V, VirenoMycin V, Albacarcin V
8-ethenyl-1-hydroxy-10,12-dimethoxy-4-(3,4,5-trihydroxy-4,6-dimethyloxan-2-yl)naphtho[1,2-c]isochromen-6-one
CHRYSOMYCIN A USP/EP/BP
6H-Benzo[d]naphtho[1,2-b]pyran-6-one, 4-(6-deoxy-3-C-methyl-β-gulopyranosyl)-8-ethenyl-1-hydroxy-10,12-dimethoxy-
82196-88-1
C28H28O9
Inhibits an EnzymeEnzyme Inhibitors by Enzyme
Topoisomerase II
A - KAntibiotics
Antibacterial
Antibiotics A to
Antibiotics A-FAntibiotics
Antibiotics by Application
Antineoplastic and Immunosuppressive AntibioticsAntibiotics
Mechanism of Action
R to
Spectrum of Activity





