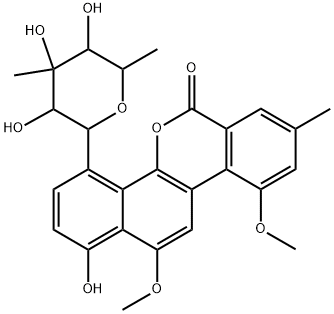
CHRYSOMYCIN B
- CAS No.
- 83852-56-6
- Chemical Name:
- CHRYSOMYCIN B
- Synonyms
- B,ChrysoMycin;CHRYSOMYCIN B USP/EP/BP;ChrysoMycin M, VirenoMycin M, Albacarcin M;6H-Benzo[d]naphtho[1,2-b]pyran-6-one, 4-(6-deoxy-3-C-methyl-β-gulopyranosyl)-1-hydroxy-10,12-dimethoxy-8-methyl-;4-(6-Deoxy-3-C-methyl-beta-gulopyranosyl)-1-hydroxy-10,12-dimethoxy-8-methyl-6H-benzo[d]naphtho[1,2-b]pyran-6-one;6H-Benzo[d]naphtho[1,2-b]pyran-6-one,4-(6-deoxy-3-C-methyl-b-gulopyranosyl)-1-hydroxy-10,12-dimethoxy-8-methyl- (9CI)
- CBNumber:
- CB3503294
- Molecular Formula:
- C27H28O9
- Molecular Weight:
- 496.51
- MOL File:
- 83852-56-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/6/30 15:45:59
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H312-H319-H351 | |||||||||
| Precautionary statements | P201-P202-P264-P270-P280-P301+P312-P330-P302+P352-P305+P351+P338-P308+P313-P321-P362+P364-P337+P313-P405-P501 | |||||||||
| WGK Germany | 3 | |||||||||
| NFPA 704 |
|
CHRYSOMYCIN B Chemical Properties,Uses,Production
Description
Chrysomycin B is an antibiotic isolated from a strain of Streptomyces. It differs from its analog chrysomycin A by having a methyl, rather than vinyl, group in the 8-position of the chromophore. Like its analog, chrysomycin B suppresses the growth of transplantable tumors in mice, an effect that may be related to its ability to bind DNA. It also causes DNA damage in the human lung adenocarcinoma A549 cell line and inhibits topoisomerase II. Chrysomycin B is structurally very similar to gilvocarcin V, which promotes DNA cross-linking with histone 3 and GRP78 when photoactivated by near-UV light.
Uses
Chrysomycin B is a minor analogue in a complex of C-glycoside antitumour actives isolated from Streptomyces. Chrysomycin B, containing a methyl group in the 8-position, is less active than its vinyl analogue (Chrysomycin A), albeit still a potent antitumour active and an inhibitor of the catalytic activity of human topoisomerase II. More recent research on related metabolites, the gilvocarcins, suggests that chrysomycins may act as photoactivated crosslinkers of DNA to histones.
CHRYSOMYCIN B Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 26165 | 58 | Inquiry |
| BOC Sciences | 16314854226; +16314854226 | United States | 19743 | 58 | Inquiry |
| TargetMol Chemicals Inc. | 15002134094 | China | 28118 | 58 | Inquiry |
| Cayman Chemical Company | (800) 364-9897 | United States | 6618 | 81 | Inquiry |
| Shanghai EFE Biological Technology Co., Ltd. | 021-65675885 18964387627 | China | 9708 | 58 | Inquiry |
| Guangzhou Isun Pharmaceutical Co., Ltd | 020-39119399 18927568969 | China | 4428 | 55 | Inquiry |
| SIGMA-RBI | 800 736 3690 (Orders) | Switzerland | 6913 | 91 | Inquiry |
| Alta Scientific Co., Ltd. | 022-6537-8550 15522853686 | China | 4515 | 55 | Inquiry |





