ChemicalBook > Product Catalog >Inorganic chemistry >Inorganic salts >Metal halide and Halogen salt >Metal fluoride and salt >HAFNIUM FLUORIDE
HAFNIUM FLUORIDE
- CAS No.
- 13709-52-9
- Chemical Name:
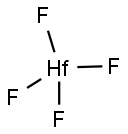
- HAFNIUM FLUORIDE
- Synonyms
- HAFNIUM FLUORIDE;HAFNIUM(IV) FLUORIDE;HAFNIUM TETRAFLUORIDE;Hafnium fluoride/ 99.9%;Hafnium(IV) fluoride,98%;HAFNIUM FLUORIDE, 99.99%;HAFNIUM TETRAFLUORIDE 99%;hafnium(4+):tetrafluoride;Hafnium tetrafluoride 99.9%;HafniuM(IV) fluoride, 98% 5GR
- CBNumber:
- CB2756332
- Molecular Formula:
- F4Hf
- Molecular Weight:
- 254.48
- MOL File:
- 13709-52-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/17 17:11:55
| Melting point | 310°C (subl.) |
|---|---|
| Boiling point | 970°C |
| Density | 7.100 |
| solubility | reacts with H2O |
| form | Powder |
| color | White |
| Water Solubility | React with water. |
| Sensitive | Moisture Sensitive |
| Exposure limits |
ACGIH: TWA 2.5 mg/m3; TWA 0.5 mg/m3 NIOSH: IDLH 250 mg/m3; IDLH 50 mg/m3; TWA 0.5 mg/m3 |
| CAS DataBase Reference | 13709-52-9(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H318-H331-H335 | |||||||||
| Precautionary statements | P280-P302+P352-P304+P340+P311-P305+P351+P338+P310 | |||||||||
| Hazard Codes | Xi,T | |||||||||
| Risk Statements | 32-36/37/38-41-23-37/38 | |||||||||
| Safety Statements | 26-36-45-36/37/39 | |||||||||
| RIDADR | UN 2923 8/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28261990 | |||||||||
| NFPA 704 |
|
HAFNIUM FLUORIDE Chemical Properties,Uses,Production
Chemical Properties
Off-white powder
Physical properties
White monoclinic crystals; refractive index 1.56; density 7.1 g/cm3; sublimes at 970°C.
Uses
Hafnium(IV) fluoride is used as Vacuum deposition.
Production Methods
Hafnium tetrafluoride is prepared by careful thermal decomposition of ammonium fluorohafniate in an oxygen- free atmosphere or by passing anhydrous hydrogen fluoride over hafnium tetrachloride at 300℃. Direct synthesis from the elements is incomplete because the product merely forms a film on the metal. Ammonium fluorohafniate can be prepared by crystallization from an aqueous hydrofluoric acid solution by adding ammonium fluoride.
HAFNIUM FLUORIDE Preparation Products And Raw materials
Raw materials
Preparation Products
Global( 89)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Alfa Chemistry | United States | 24072 | 58 | Inquiry | |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 8672 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 50003 | 58 | Inquiry |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | China | 33350 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 57511 | 58 | Inquiry |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | China | 40067 | 58 | Inquiry |
| ABCR GmbH & CO. KG | 49 721 95061 0 | Germany | 6846 | 75 | Inquiry |
| ProChem, Inc. | 815 398 1788 | United States | 1692 | 66 | Inquiry |
| Noah Chemicals | (888) 291-1186 | United States | 881 | 58 | Inquiry |
13709-52-9(HAFNIUM FLUORIDE)Related Search:
Hafnium(IV) fluoride, 99.9% trace metals basis excluding Zr
HAFNIUM FLUORIDE
HAFNIUM(IV) FLUORIDE
HAFNIUM TETRAFLUORIDE
HAFNIUM TETRAFLUORIDE 99%
HAFNIUM FLUORIDE, 99.99%
Hafnium tetrafluoride 99.9%
Hafnium(IV) fluoride, 99.9% (metals basis excluding Zr), Zr 2-4%
HAFNIUM (IV) FLUORIDE 99.9% (METALS)
Hafnium(IV) fluoride, 99.9% (metals basis)
Hafnium(IV) fluoride,98%
Hafnium fluoride/ 99.9%
HafniuM(IV) fluoride, 98% 5GR
hafnium(4+):tetrafluoride
HAFNIUM FLUORIDE, 99.99%HAFNIUM FLUORIDE, 99.99%HAFNIUM FLUORIDE, 99.99%HAFNIUM FLUORIDE, 99.99%
Hafnium fluoride (HfF4), (T-4)-
Gadolinium Oxide (Gd2O3) Sputtering Targets
13709-52-9
HfF4
F4Hf
Hafnium Salts
Metal and Ceramic Science
Salts
Hafnium Salts
Materials Science
Metal and Ceramic Science





