Hydrogen fluoride
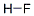
- CAS No.
- 7664-39-3
- Chemical Name:
- Hydrogen fluoride
- Synonyms
- HF;HYDROFLUORIC ACID;Anhydrous hydrofluoric acid;hydrofluoric;Urea Hydrofluoride;HydrofL;Fluoric acid;Hydrofluoride;Hydrofluoric acid(HF);Hydrofluoric Acid, TraceGrade
- CBNumber:
- CB8380315
- Molecular Formula:
- FH
- Molecular Weight:
- 20.01
- MOL File:
- 7664-39-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/14 15:18:27
| Melting point | -35°C |
|---|---|
| Boiling point | 105°C |
| Density | 1.15 g/mL at 25 °C(lit.) |
| vapor density | 1.27 (vs air) |
| vapor pressure | 25 mm Hg ( 20 °C) |
| Flash point | 112°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | very soluble in H2O, ethanol; soluble in ethyl ether |
| pka | 3.17(at 25℃) |
| form | Liquid, Double Sub-Boiling Quartz Distillation |
| Specific Gravity | 1.15 |
| Odor | Acrid, irritating odor |
| PH Range | 1 |
| PH | 3.27(1 mM solution);2.65(10 mM solution);2.12(100 mM solution) |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,4790 |
| Exposure limits | Ceiling limit 3 ppm (~2.5 mg/m3) as F (ACGIH); TWA 3 ppm (MSHA and OSHA). |
| Dielectric constant | 17.0(-73℃) |
| Stability | Stable. Hygroscopic. Incompatible with glass, alkali metals, light metals, alkaline earth metals |
| LogP | 0.1 at 20℃ |
| CAS DataBase Reference | 7664-39-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Hydrogen fluoride(7664-39-3) |
| EPA Substance Registry System | Hydrofluoric acid (7664-39-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310+H330-H314 | |||||||||
| Precautionary statements | P260-P270-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | T+,C,T,Xn | |||||||||
| Risk Statements | 26/27/28-35-36/37/38-20/21/22 | |||||||||
| Safety Statements | 26-36/37/39-45-7/9-36/37-28-36 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 3 ppm (2.5 mg/m3), Ceiling: 6 ppm (5 mg/m3) [15-minute] | |||||||||
| RIDADR | UN 1790 8/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | MW7875000 | |||||||||
| Hazard Note | Corrosive | |||||||||
| TSCA | Yes | |||||||||
| DOT Classification | 8, Hazard Zone C (Corrosive material) | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28111100 | |||||||||
| Toxicity | LC50 (15 min.) in rats, guinea pigs: 2689, 4327 ppm (Rosenholtz) | |||||||||
| IDLA | 30 ppm | |||||||||
| NFPA 704 |
|
Hydrogen fluoride price More Price(27)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 339261 | Hydrofluoric acid 48?wt. % in H2O, ≥99.99% trace metals basis | 7664-39-3 | 100ML | ₹11041.5 | 2022-06-14 | Buy |
| Sigma-Aldrich | 339261 | Hydrofluoric acid 48?wt. % in H2O, ≥99.99% trace metals basis | 7664-39-3 | 100ML | ₹11041.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 339261 | Hydrofluoric acid 48?wt. % in H2O, ≥99.99% trace metals basis | 7664-39-3 | 100ML | ₹11041.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 695068 | Hydrofluoric acid ACS reagent, 48% | 7664-39-3 | 25ML | ₹4037.73 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 339261 | Hydrofluoric acid 48?wt. % in H2O, ≥99.99% trace metals basis | 7664-39-3 | 4X100ML | ₹23706.75 | 2022-06-14 | Buy |
Hydrogen fluoride Chemical Properties,Uses,Production
Description
Hydrofluoric acid is a solution of hydrogen fluoride in water. Hydrofluoric acid is highly corrosive inorganic acid. It is utilized widely in the manufacture of ceramics and graphite, in the electropolishing and pickling of metals, in the etching and frosting of glass, in the semiconductor industry as etchant and cleaning agent, in the chemical and oil-refining industries, and in cleaning solutions, laundry powder and pesticides. Hydrofluoric acid is also widely used in the preparation of many useful fluorine compounds, such as Teflon, Freon, fluorocarbons, and many medications such as fluoxetine (Prozac).
Chemical Properties
colourless gas with a pungent odour
Uses
Hydrofluoric acid is used as a fluorinatingagent, as a catalyst, and in uranium refining.It is also used for etching glass and forpickling stainless steel. Hydrogen fluoridegas is produced when an inorganic fluoride is distilled with concentrated sulfuricacid.
Production Methods
Anhydrous hydrogen fluoride is manufactured by the action of sulfuric on calcium fluoride. Powdered acid-grade fluorspar (≥97% CaF2) is distilled with concentrated sulfuric acid; the gaseous hydrogen fluoride that leaves the reactor is condensed and purified by distillation.
Anhydrous hydrogen fluoride is manufactured by treating fluorspar (fluorite, CaF2) with concentrated sulfuric acid in heated kilns. The gaseous HF evolved is purified by distillation, condensed as liquid anhydrous HF, and stored in steel tanks and cylinders.
Definition
A colorless liquid produced by dissolving hydrogen fluoride in water. It is a weak acid, but will dissolve most silicates and hence can be used to etch glass. As the interatomic distance in HF is relatively small, the H–F bond energy is very high and hydrogen fluoride is not a good proton donor. It does, however, form hydrogen bonds.
General Description
HF is a colorless inorganic acid. Hydrogen fluoride may be formed by reacting calcium fluoride and sulfuric acid at 200oC. The fluoride in the acid has very high affinity to silicon, making it useful in etching or removal of silicon.
Air & Water Reactions
Fumes in air. Fumes are highly irritating, corrosive, and poisonous. Generates much heat on dissolution [Merck, 11th ed., 1989]. Heat can cause spattering, fuming, etc.
Reactivity Profile
Hydrofluoric acid attacks glass and any other silica containing material. May react with common metals (iron, steel) to generate flammable hydrogen gas if diluted below 65% with water. Reacts exothermically with chemical bases (examples: amines, amides, inorganic hydroxides). Can initiate polymerization in certain alkenes. Reacts with cyanide salts and compounds to release gaseous hydrogen cyanide. May generate flammable and/or toxic gases with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides. Additional gas-generating reactions may occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and carbonates. Can catalyze (increase the rate of) chemical reactions. Reacts explosively with cyanogen fluoride, methanesulfonic acid or glycerol mixed with nitric acid. Reacts violently with arsenic trioxide, phosphorus pentachloride, acetic anhydride, alkali metals, ammonium hydroxide, chlorosulfonic acid, ethylenediamine, fluorine, potassium permanganate, oleum, propylene oxide, vinyl acetate, mercury(II) oxide. Emits highly corrosive fumes of hydrogen fluoride gas when heated [Sax, 9th ed., 1996, p. 1839]. Contact with many silicon compounds and metal silicides causes violent evolution of gaseous silicon tetrafluoride [Mellor, 1956, Vol. 2, suppl. 1, p. 121].
Hazard
Toxic by ingestion and inhalation, highly corrosive to skin and mucous membranes.
Health Hazard
Hydrofluoric acid and hydrogen fluoride gasare extremely corrosive to body tissues, causing severe burns. The acid can penetrate theskin and destroy the tissues beneath and evenaffect the bones. Contact with dilute acid cancause burns, which may be perceptible hoursafter the exposure. The healing is slow. Contact with the eyes can result in impairment ofvision.
Prolonged exposure to 10–15 ppm concentrations of the gas may cause redness ofskin and irritation of the nose and eyes inhumans. Inhalation of high concentrations ofHF may produce fluorosis and pulmonaryedema. In animals, repeated exposure toHF gas within the range 20–25 ppm hasproduced injury to the lungs, liver, andkidneys.
LC50 value, inhalation (mice): 342 ppm/h.
Fire Hazard
When heated, Hydrofluoric acid emits highly corrosive fumes of fluorides. Its corrosive action on metals can result in formation of hydrogen in containers and piping to create fire hazard. Toxic and irritating vapors are generated when heated. Will attack glass, concrete, and certain metals, especially those containing silica, such as cast iron. Will attack natural rubber, leather, and many organic materials. May generate flammable hydrogen gas in contact with some metals.
Flammability and Explosibility
Hydrogen fluoride is not a combustible substance
Industrial uses
Hydrofluoric acid (HF) is a colorless liquid with a characteristic odor. It releases fumes
when in contact with moist air. Hydrofluoric acid is manufactured from fluorite containing
96–97% CaF2 by reacting it with concentrated sulfuric acid:
CaF2+H2SO4 = 2HF+CaSO4
The acid is sold as a 40% solution. The hydrofluoric acid is used as an activator and
depressant, mostly during flotation of industrial minerals (i.e. columbite, tantalite,
silica, feldspars).
Materials Uses
Carbon steel (without nonmetallic inclusions) is acceptable for handling hydrogen fluoride up to approximately 150°F (65.6°C). Aluminum- silicon-bronze, stainless steel, or nickel are suitable for cylinder valves. For higher temperatures, Monel, Inconel, nickel, or copper should be used. Cast iron or malleable fittings should be avoided. Polyethylene, lead, soft copper, Kel-F, and Teflon are acceptable gasket materials. Polyethylene, Kel-F, and Teflon are acceptable packing materials.
Carcinogenicity
NTP conducted two chronic oral bioassays of fluoride administered as sodium fluoride (0, 25, 100, or 175 ppm) in drinking water for 103 weeks in rats and mice.The first study was compromised, so it was used to determine doses for the second study. NTP concluded that there was no evidence that fluoride was carcinogenic at doses up to 4.73 mg/kg/day in female rats or at doses up to 17.8 and 19.9 mg/kg/day in male and female mice, respectively.
Environmental Fate
Hydrogen fluoride is a colorless, fuming liquid with a strong,
irritating odor. The density is 1.002 at 0 ℃ and the boiling
point is 19.51 ℃. Hydrogen Fluoride is naturally released into
the environment, primarily from volcanoes, ranging from 0.6
to 6 million metric tons per year. The majority of artificial
pollutants come from electrical utilities.
Hydrogen fluoride is removed from air by wet deposition as
fluoride salts with an atmospheric lifetime of 1–5 days.
storage
All work with HF should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and neoprene gloves should be worn at all times to prevent eye and skin contact. Containers of HF should be stored in secondary containers made of polyethylene in areas separate from incompatible materials. Work with anhydrous HF should be undertaken using special equipment and only by well-trained personnel familiar with first aid procedures.
Purification Methods
It can be purified by trap-to-trap distillation, followed by drying over CoF2 at room temperature and further distillation. Alternatively, it can be absorbed on NaF to form NaHF2 which is then heated under vacuum at 150o to remove volatile impurities. The HF is regenerated by heating at 300o and is stored with CoF3 in a nickel vessel, being distilled as required. (Water content should be ca 0.01%.) To avoid contact with base metal, use can be made of nickel, polychlorotrifluoroethylene and gold-lined fittings [Hyman et al. J Am Chem Soc 79 3668 1957]. An aqueous solution is hydrofluoric acid (see above). It is HIGHLY TOXIC and attacks glass.
Incompatibilities
HF reacts with glass, ceramics, and some metals. Reactions with metals may generate potentially explosive hydrogen gas.
Waste Disposal
Excess hydrogen fluoride and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines. For more information on disposal procedures, see Chapter 7 of this volume.
References
[1] David J. Monk, and David S. Soane, A review of the chemical reaction mechanism and kinetics for hydrofluoric acid etching of silicon dioxide for surface micromachining applications, Thin Solid Films, 1993, vol. 232, 1-12
[2] P. Sanz-Gallen, S. Nogue, P. Munne and A. Faraldo, Hybocalcaemia and hypomagnesaemia due to hydrofluoric acid, Occup Med (Lond), 2001, vol. 51, 294-295
Hydrogen fluoride Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| oswal chemicals | +91-9173644055 +91-9173644055 | Gujarat, India | 66 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Gajanan Enterprises | +91-8422892201 +91-8422892201 | Maharashtra, India | 8 | 58 | Inquiry |
| Merck Ltd | +91-2262109800 +91-2262109000 | Maharashtra, India | 272 | 58 | Inquiry |
| Navin Fluorine International Ltd | +91-2266509999 +91-2266509999 | West Bengal, India | 7 | 58 | Inquiry |
| Sri Chemicals | +91-4426207575 +91-4442127722 | Tamil Nadu, India | 21 | 58 | Inquiry |
| Sima Chemicals (Sima Products) | +91-9821136312 +91-9321375313 | Maharashtra, India | 20 | 58 | Inquiry |
| S D Fine Chem Limited | +91-9323715856 +91-9323715856 | Maharashtra, India | 368 | 58 | Inquiry |
| LANXESS India Pvt. Ltd. | +91-8356878763 +91-8356878763 | Maharashtra, India | 190 | 58 | Inquiry |
| Ennor Muds and Chemicals | +91-8754442175 +91-9840477722 | Tamil Nadu, India | 26 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| oswal chemicals | 58 |
| JSK Chemicals | 58 |
| Gajanan Enterprises | 58 |
| Merck Ltd | 58 |
| Navin Fluorine International Ltd | 58 |
| Sri Chemicals | 58 |
| Sima Chemicals (Sima Products) | 58 |
| S D Fine Chem Limited | 58 |
| LANXESS India Pvt. Ltd. | 58 |
| Ennor Muds and Chemicals | 58 |
Related articles
- Overview of hydrogen fluoride polarity
- Hydrogen fluoride (fluorane) is an inorganic compound with chemical formula HF. It is a very poisonous, colorless gas or liqui....
- Dec 19,2023
- Uses and production of Hydrogen fluoride
- Hydrogen fluoride is a colorless, corrosive liquid or gas and is composed of a hydrogen atom and a fluorine atom.
- Apr 20,2022
- Hydrofluoric acid-Hazard and Toxicity
- Hydrofluoric acid (HF) is an aqueous solution of hydrogen fluoride gas. At room temperature, it appears as colorless transpare....
- Sep 9,2019
7664-39-3(Hydrogen fluoride)Related Search:
1of4
chevron_right




