Doxycycline
- CAS No.
- 564-25-0
- Chemical Name:
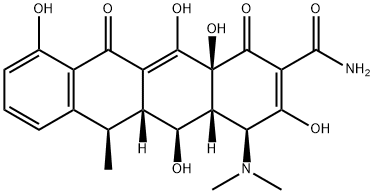
- Doxycycline
- Synonyms
- doxycyline;vibramycin;doxiciclina;Doxycyclin hyclate;DOXYCYCLINE HYDRATE;doryx;liviatin;Doxycyclinum;(4S,4aR,5S,5aR,6R,12aS)-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide;(4S,4aR,5S,5aR,6R,12aS)-4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide
- CBNumber:
- CB8663342
- Molecular Formula:
- C22H24N2O8
- Molecular Weight:
- 444.43
- MOL File:
- 564-25-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/2 18:12:07
| Melting point | 206-209°C (dec.) |
|---|---|
| Boiling point | 554.44°C (rough estimate) |
| Density | 1.3809 (rough estimate) |
| refractive index | 1.6500 (estimate) |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | DMSO : 125 mg/mL (281.26 mM; Need ultrasonic) |
| pka | pKa 3.5 (Uncertain);7.7 (Uncertain);9.5 (Uncertain) |
| form | Solid |
| color | Light yellow to brown |
| Water Solubility | 0.63g/L(25 ºC) |
| BCS Class | 3,1 |
| InChIKey | JBIWCJUYHHGXTC-AKNGSSGZSA-N |
| SMILES | C1(=O)[C@]2(O)[C@@]([H])([C@@H](O)[C@@]3([H])C(=C2O)C(=O)C2=C(C=CC=C2O)[C@@H]3C)[C@H](N(C)C)C(O)=C1C(N)=O |
| CAS DataBase Reference | 564-25-0(CAS DataBase Reference) |
| EPA Substance Registry System | Doxycycline (564-25-0) |
Doxycycline Chemical Properties,Uses,Production
Chemical Properties
Yellow Solid
Uses
Doxycycline is a semi-synthetic tetracycline prepared by hydrogenolysis of oxytetracycline to remove the 6-hydroxy group. Although the synthesis was reported in 1958, it was not released for use until 1967. Doxycycline, together with minocycline, is regarded as a ‘third generation’ tetracycline largely replacing the analogues and pro-drugs produced in the early 1960s for mainstream antibiotic applications. Like all tetracyclines, doxycycline shows broad spectrum antibacterial and antiprotozoan activity and acts by binding to the 30S and 50S ribosomal subunits, blocking protein synthesis. Doxycycline has been extensively cited in the literature with over 10,000 references.
Definition
ChEBI: Tetracycline in which the 5beta-hydrogen is replaced by a hydroxy group, while the 6alpha-hydroxy group is replaced by hydrogen. A semi-synthetic tetracycline antibiotic, it is used to inhibit bacterial protein synthesis a d treat non-gonococcal urethritis and cervicitis, exacerbations of bronchitis in patients with chronic obstructive pulmonary disease (COPD), and adult periodontitis.
Indications
Doxycycline (Vibramycin, Monodox) has similar absorption and durationof- activity characteristics. Its effectiveness in acne approaches that of minocycline, when used in the same fashion with similar dosages. Early data suggests that subantimicrobial doses of doxycycline, 20 mg (Periostat), may play a therapeutic role in acne by reducing inflammation through anticollagenolytic, antimatrix-degrading metalloproteinase, and cytokine downregulating properties.
Antimicrobial activity
It is active against some tetracycline-resistant Staph. aureus and is more active than other tetracyclines against Str. pyogenes, enterococci and Nocardia spp. Mor. catarrhalis (MIC 0.5 mg/L), Legionella pneumophila and most strains of Ureaplasma urealyticum (MIC 0.5 mg/L) are susceptible.
Pharmaceutical Applications
6-Deoxy-5β-hydroxytetracycline. A semisynthetic product supplied as the hyclate, calcium salt or the hydrochloride for oral and intravenous administration.
Clinical Use
Like the other tetracyclines, doxycycline inhibits the pathogen’s protein synthesisby reversibly inhibiting the 30S ribosomal subunit.Bacteria and Plasmodium ribosomal subunits differ significantlyfrom mammalian ribosomes such that this group ofantibiotics do not readily bind to mammalian ribosomesand, therefore, show good selective toxicity. Althoughdoxycycline is a good antibacterial, its use for malaria islimited to prophylaxis against strains of P. falciparumn resistantto chloroquine and sulfadoxine–pyrimethamine.This use normally should not exceed 4 months. Becausethe tetracyclines chelate calcium, they can interfere withdevelopment of the permanent teeth in children. Therefore,their use in children definitely should be short term. Also, tetracycline photosensitivity must be kept in mind, particularlybecause areas where malaria is endemic are also theareas with the greatest sunlight.
Side effects
Untoward reactions are generally those typical of the group
but gastrointestinal side effects are less common than with
other tetracyclines due to the lower total dosage and the ability
to administer the drug with meals. Esophageal ulceration
as a result of capsule impaction has been reported. Dental and
bone deposition appear to be less common than with other
tetracycline derivatives. Other adverse phenomena include
occasional vestibular toxicity.
Hypersensitivity reactions include photosensitivity and
eosinophilia, but rarely anaphylaxis. In common with demeclocycline
and chlortetracycline it may be a more powerful
sensitizer than other tetracyclines. It is contraindicated in
patients with acute porphyria because it has been demonstrated
to be porphyrinogenic in animals.
Metabolism
Doxycycline is well absorbed on oral administration (90–100% when fasting; reduced by 20% by co-consumption with food or milk), has a half-life permitting once-a-day dosing for mild infections, and is excreted partly in the feces and partly in the urine.
Doxycycline Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CHEMXTREE STANDARDS | +919872732255 | Punjab, India | 96 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| PROTECH TELELINKS | +91-8571891912 +91-9855060837 | Himachal Pradesh, India | 62 | 58 | Inquiry |
| Vijaya Pharma And Life Science | +91-8939866271 +91-8939866271 | Tamil Nadu, India | 320 | 58 | Inquiry |
| Maithri Drugs Pvt Ltd | +91-9059204566 +91-9848881740 | Telangana, India | 64 | 58 | Inquiry |
| Raks Pharma Pvt Ltd | +91-8924671555 +91-8924671555 | AndhraPradesh, India | 31 | 58 | Inquiry |
| AKASH PHARMA EXPORTS | +91-9388123451 +91-9846039283 | Kerela, India | 470 | 58 | Inquiry |
| RAKS PHARMA PVT LTD | +91 891 3081555 | New Delhi, India | 65 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| CHEMXTREE STANDARDS | 58 |
| Ralington Pharma | 58 |
| PROTECH TELELINKS | 58 |
| Vijaya Pharma And Life Science | 58 |
| Maithri Drugs Pvt Ltd | 58 |
| Raks Pharma Pvt Ltd | 58 |
| AKASH PHARMA EXPORTS | 58 |
| RAKS PHARMA PVT LTD | 58 |
| Pharmaffiliates Analytics and Synthetics P. Ltd | 58 |
| SynZeal Research Pvt Ltd | 58 |
Related articles
- A second-generation tetracycline---Doxycycline
- Doxycycline (alpha-6-deoxytetracycline) is a second-generation tetracycline with increased oral bioavailability and tissue pen....
- Mar 17,2022
- What to know about doxycycline
- Doxycycline may not be appropriate for everyone. It may interact with some other drugs and can cause side effects. Doctors wil....
- Oct 21,2019
564-25-0(Doxycycline)Related Search:
1of4
chevron_right




