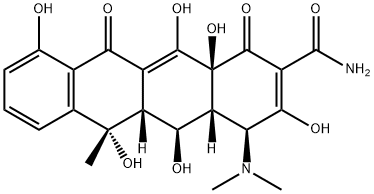
Oxytetracycline
- CAS No.
- 79-57-2
- Chemical Name:
- Oxytetracycline
- Synonyms
- OXYTETRACYCLINE BASE;Terramycin;oxytetracyclin;oxy-kesso-tetra;Oxytetracycline CRS;4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxo-, 2-Naphthacenecarboxamide;la200;Oxydon;oxypam;tetran
- CBNumber:
- CB6429340
- Molecular Formula:
- C22H24N2O9
- Molecular Weight:
- 460.43
- MOL File:
- 79-57-2.mol
- Modify Date:
- 2024/6/11 18:14:38
| Melting point | 183 °C |
|---|---|
| alpha | -223 º (c=1 0.03N HCl) |
| Boiling point | 565.29°C (rough estimate) |
| Density | 1.6340 |
| refractive index | 1.6500 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Sparingly soluble in aqueous solution at 0.6 mg/mL |
| form | Crystalline Powder |
| pka | pKa 3.27/7.32/9.11(H2O,t =25,I<0.01)(Approximate) |
| color | Beige to light yellow |
| Water Solubility | 0.2 g/L |
| InChIKey | IWVCMVBTMGNXQD-PXOLEDIWSA-N |
| LogP | -0.900 |
| CAS DataBase Reference | 79-57-2(CAS DataBase Reference) |
| EPA Substance Registry System | Oxytetracycline (79-57-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H361d-H411 | |||||||||
| Precautionary statements | P201-P202-P273-P280-P308+P313-P391 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 63-36-36/37/38 | |||||||||
| Safety Statements | 22-24/25-36/37/39-26-36 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | QI7875000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 30029090 | |||||||||
| Toxicity | LD50 oral in rat: 4800mg/kg | |||||||||
| NFPA 704 |
|
Oxytetracycline Chemical Properties,Uses,Production
Description
Oxytetracycline also is one of the classic tetracyclines. It is produced by fermentation of Streptomyces rimosis and other soil microorganisms. The most hydrophilic tetracycline on the market, it has largely now been replaced by its semisynthetic descendants. It is primarily used today for IM injections.
Chemical Properties
beige to light yellow crystalline powder
Uses
Oxytetracycline(Terramycin) was the second of the broad-spectrum tetracycline group of antibiotics to be discovered. Oxytetracycline works by interfering with the ability of bacteria to produce proteins that are essential to them. Without these proteins t
Definition
ChEBI: A tetracycline used for treatment of infections caused by a variety of Gram positive and Gram negative microorganisms including Mycoplasma pneumoniae, Pasteurella pestis, Escherichia coli, Haemophilus influenzae /ital> (respiratory infections), and Diplococcus pneumoniae.
Antimicrobial activity
It is slightly less active than other tetracyclines against most common pathogenic bacteria.
Pharmaceutical Applications
A fermentation product of certain strains of Streptomyces rimosus, supplied as the dihydrate or hydrochloride for oral or parenteral administration.
Pharmacokinetics
Oral absorption: c.60%
Cmax 500 mg oral: 3–4 mg/L after 2–4 h
Plasma half-life: c.9 h
Volume of distribution: c.1.8 L/kg
Plasma protein binding: 20–35%
Oxytetracycline is moderately well absorbed from the upper
gastrointestinal tract. Food decreases plasma levels by approximately
50%. Although widely distributed in the tissues, it achieves lower concentrations than related agents such as minocycline.
Sputum concentrations of 1 mg/L have been recorded
on a daily dosage of 2 g. Approximately 60% is excreted in the
urine and the half-life is prolonged in renal insufficiency.
Clinical Use
It offers no unique therapeutic advantages, although it is one of the cheaper preparations.
Side effects
Gastrointestinal intolerance is responsible for most side effects, and tends to be more severe than with other tetracyclines. Esophageal irritation may result from the local effects of the swallowed drug. Potentially serious adverse reactions have included neuromuscular paralysis following intravenous administration to patients with myasthenia gravis. Thrombocytopenic purpura and lupus erythematosus syndrome have been reported, although a direct role for the drug in the latter remains uncertain. Apart from the effect on nitrogen balance common to many tetracyclines, a metabolic effect on glucose homeostasis has been noted in type 1 diabetes mellitus. Allergic contact sensitivity reactions have also been reported.
Purification Methods
Terramycin crystallises (as dihydrate) from water or aqueous EtOH and is soluble in MeOH, EtOH, Me2CO and H2O (0.25mg/mL at 25o ) but insoluble in Et2O and pet ether. It is amphoteric, and an aqueous solution has pH 2.0-5.0. It has max at 247, 275 and 353nm in 0.1 M phosphate (pH 4.5). [Finlay et al. Science 111 85 1951.] The hydrochloride, [2058-46-0] M 496.9 [Beilstein 14 IV 2630], crystallises from MeOH in needles and from H2O at 50o it forms plates. Terramycin has also been purified via the hydrochloride by dissolving it in H2O, adjusting to pH 6, and the solid is filtered off after 1hour. The crystals of the dihydrate are dried to constant weight in vacuum/CaCl2/25o. Drying at 60o in vacuo gives the anhydrous base m 184.5-185.5o (sintering at 180o). The dihydrate has m 181-182o. Its optical rotation in MeOH decreases from [] D 25 +26o (c 0.5%) to [] D 25 +11.3o after standing for 16hours. It forms a sodium salt and a CaCl2 complex. [Regna et al. J Am Chem Soc 73 4211 1951, Beilstein 14 IV 2633.]
Oxytetracycline Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Molsyns Research | +91-9586858886 +91-9586858886 | Gujarat, India | 418 | 58 | Inquiry |
| Century Pharmaceuticals Ltd | +91-2652361581 +91-2652361581 | Gujarat, India | 53 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| L S Healthcare | 08048250308Ext 389 | Mumbai, India | 12 | 58 | Inquiry |
| Rhyme Organics And Chemicals Limited | 08048372046Ext 929 | Hyderabad, India | 102 | 58 | Inquiry |
| Drugs India Mahaveeray | 08069033817Ext 926 | Hyderabad, India | 1 | 58 | Inquiry |
| Utkarsh Biotech | 08048611095 | Mumbai, India | 16 | 58 | Inquiry |
79-57-2(Oxytetracycline)Related Search:
1of4
chevron_right




