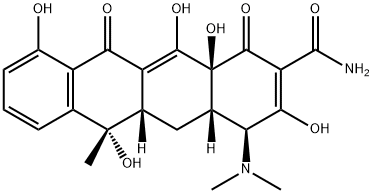
Tetracycline
- CAS No.
- 60-54-8
- Chemical Name:
- Tetracycline
- Synonyms
- TETRACYCLIN;4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-Naphthacenecarboxamide;Limecycline;TETRACYCLINE BASE;panmycin;tetracyn;bristacycline;t-125;cytome;amycin
- CBNumber:
- CB6153810
- Molecular Formula:
- C22H24N2O8
- Molecular Weight:
- 444.43
- MOL File:
- 60-54-8.mol
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 175-177 °C(lit.) |
|---|---|
| alpha | D25 -257.9° (0.1N HCl); D25 -239° (methanol) |
| Boiling point | 554.44°C (rough estimate) |
| Density | 1.3809 (rough estimate) |
| refractive index | 1.6500 (estimate) |
| storage temp. | 2-8°C |
| solubility | 95% ethanol: soluble12.5mg/mL |
| form | powder |
| pka | pKa (50% aq DMF): 8.3, 10.2(at 25℃) |
| color | yellow to yellow orange |
| optical activity | [α]25/D -239° in methanol (Specific rotation ) &_& [α]25/D -257.9°, c = 0.1 M HCl mol (Specific rotation ) |
| Water Solubility | Limited solubility in water. Soluble in 1M HCl with heating. |
| Merck | 13,9271 |
| BRN | 2230417 |
| BCS Class | 3 |
| Stability | Hygroscopic |
| InChIKey | OFVLGDICTFRJMM-WESIUVDSSA-N |
| CAS DataBase Reference | 60-54-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Tetracycline(60-54-8) |
| EPA Substance Registry System | Tetracycline (60-54-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H361fd-H411 |
| Precautionary statements | P202-P264-P270-P273-P301+P312-P308+P313 |
| Hazard Codes | Xn,Xi |
| Risk Statements | 22-36/37/38 |
| Safety Statements | 22-36-26 |
| WGK Germany | 3 |
| RTECS | QI8750000 |
| F | 3-8-10 |
| HS Code | 29413000 |
| Hazardous Substances Data | 60-54-8(Hazardous Substances Data) |
| Toxicity | LD50 in rats, mice (mg/kg): 807, 808 orally (Goldenthal) |
Tetracycline price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | T3258 | Tetracycline 98.0-102.0% (HPLC) | 60-54-8 | 5G | ₹2641.3 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T3258 | Tetracycline 98.0-102.0% (HPLC) | 60-54-8 | 25G | ₹6992.95 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T3258 | Tetracycline 98.0-102.0% (HPLC) | 60-54-8 | 100G | ₹10922.43 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 87128 | Tetracycline 98.0-102.0% (HPLC) | 60-54-8 | 25G | ₹6852.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 87128 | Tetracycline 98.0-102.0% (HPLC) | 60-54-8 | 100G | ₹18629.83 | 2022-06-14 | Buy |
Tetracycline Chemical Properties,Uses,Production
Description
Tetracycline is an antibiotic that has been utilized in disease management situations in which SmR strains of E. amylovora or P. syringae already exist. However, tetracycline does not appear to be as effective as streptomycin in reducing blossom populations of E. amylovora (17). Additionally, strains of P. syringae with resistance to tetracycline have been isolated from pear orchards in Oregon and Washington (18), suggesting that resistance to this antibiotic will probably develop in orchards where it is applied.
Chemical Properties
Tetracycline trihydrate is a white crystalline substance.
Uses
Tetracycline is a linear, tetracyclic, broad spectrum antibiotic first prepared chemically by dechlorination of chlortetracycline and subsequently isolated from several Streptomyces species. Tetracycline has broad spectrum antibacterial and antiprotozoan activity, and acts by binding to the 30S and 50S ribosomal subunits blocking protein synthesis. Tetracycline is a pigment and, like many pigments, is degraded by light, oxygen, trace metal ions and pH variations. The purity of tetracycline is often variable, with significant levels of degradation products.
Indications
Tetracycline is often the first antibiotic prescribed. It is the least expensive, has few side effects, and is well tolerated for longer periods of time. Tetracycline is effective in low doses because high concentrations are achieved within sebaceous follicles, especially when inflammation is present.
World Health Organization (WHO)
The first tetracycline antibiotic, chlortetracycline, was introduced in 1948 and subsequently several semisynthetic derivatives have been used as antibacterial, antiamoebic and antirickettsial agents. All tetracyclines accumulate in the developing bones and teeth of the foetus and young children which can result in retarded bone growth and dental staining. Preparations intended specifically for children have been withdrawn in some countries, whereas in others warnings are required on the label advising against administration of tetracyclines to young children and pregnant women. Non-paediatric dosage forms of tetracycline remain in the WHO Model List of Essential Drugs.
Antimicrobial activity
It is also active against V. cholerae, chlamydiae, rickettsiae and spirochetes.
General Description
Chemical studies on chlortetracycline revealed that controlledcatalytic hydrogenolysis selectively removed the 7-chloro atom and so produced tetracycline (Achromycin,Cyclopar, Panmycin, Tetracyn). This process was patentedby Conover in 1955. Later, tetracycline was obtainedfrom fermentations of Streptomyces spp., but the commercialsupply still chiefly depends on hydrogenolysis of chlortetracycline.
Tetracycline is 4-dimethyl amino-1,4,4a,5,5a,6,11,12aoctahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide. It is a bright yellow, crystallinesalt that is stable in air but darkens on exposure tostrong sunlight. Tetracycline is stable in acid solutions witha pH above 2. It is somewhat more stable in alkaline solutionsthan chlortetracycline, but like those of the other tetracyclines,such solutions rapidly lose potency. One gram ofthe base requires 2,500 mL of water and 50 mL of alcohol todissolve it. The hydrochloride salt is used most commonly inmedicine, though the free base is absorbed from the GI tractabout equally well. One gram of the hydrochloride salt dissolvesin about 10 mL of water and in 100 mL of alcohol.Tetracycline has become the most popular antibiotic of itsgroup, largely because its plasma concentration appears to behigher and more enduring than that of either oxytetracyclineor chlortetracycline. Also, it is found in higher concentrationin the spinal fluid than the other two compounds.
Pharmaceutical Applications
A fermentation product of Streptomyces aureofaciens, also produced from chlortetracycline. Available as the hydrochloride for oral and topical use.
Clinical Use
Along with doxycycline it is one of the most commonly used tetracyclines.
Potential Exposure
Tetracycline is an antibiotic medicine used as capsules, tablets, or intravenous injections against certain infections in humans and animals.
Shipping
UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Tetracycline crystallises from toluene or aqueous MeOH as the trihydrate. [Stephen et al. J Am Chem Soc 76 3568 1954, Beilstein 14 IV 2625.]
Incompatibilities
Although no dangerous incompatibilities are reported, the potency of this medicine is reduced by heat, sunlight, and solutions with pH <2; and destroyed by caustic hydroxide solutions.
Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
Tetracycline Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| KARPSCHEM LABORATORIES | +91-7249203006 +91-7249203006 | Maharashtra, India | 786 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6514 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6088 | 58 | Inquiry |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 | China | 8808 | 58 | Inquiry |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | China | 12809 | 58 | Inquiry |
| Hangzhou ICH Biofarm Co., Ltd | +86-0571-28186870; +undefined8613073685410 | China | 1015 | 58 | Inquiry |
| Shaanxi Xianhe Biotech Co., Ltd | +8617709210191 | China | 884 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21628 | 55 | Inquiry |
Related articles
- Mechanism of action of Tetracycline
- Tetracycline was first described in 1953 and was prepared from chlortetracycline at Lederle Laboratories, and was also indepen....
- Mar 17,2022
60-54-8(Tetracycline)Related Search:
1of4
chevron_right




