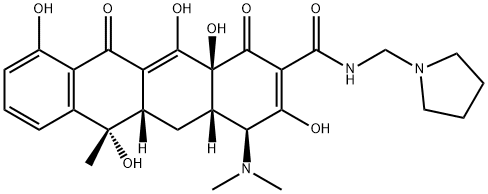
ROLITETRACYCLINE
- CAS No.
- 751-97-3
- Chemical Name:
- ROLITETRACYCLINE
- Synonyms
- prm-tc;reverin;sq15,659;bristacin;syntetrex;syntetrin;Syntodecin;Tetraverin;superciclin;synotodecin
- CBNumber:
- CB7666669
- Molecular Formula:
- C27H33N3O8
- Molecular Weight:
- 527.57
- MOL File:
- 751-97-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/6/8 9:02:53
| Melting point | 163.5°C |
|---|---|
| Boiling point | 608.16°C (rough estimate) |
| Density | 1.2902 (rough estimate) |
| refractive index | 1.7120 (estimate) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Very Slightly) |
| form | Solid |
| pka | pKa 7.4 (Uncertain) |
| color | Very Dark Red to Very Dark Brown |
| Water Solubility | >20g/L(21 ºC) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P305+P351+P338 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | QI9150000 | |||||||||
| NFPA 704 |
|
ROLITETRACYCLINE Chemical Properties,Uses,Production
Description
Rolitetracycline was synthesized in 1958. Bristol-Myers Laboratories prepared it from tetracycline by introducing a pyrrolidinylmethyl moiety. This antibiotic is very soluble in water and more stable than tetracycline under acidic conditions. Rolitetracycline is used by intravenous injection, and its nitrate is used by both intravenous and intramuscular injections for therapy of the same infections as those treated by tetracycline.
Uses
Rolitetracycline is a tetracycline antibiotic that may be given intravenously or intramuscularly in serious bacterial infections when oral administration is not practicable.
Definition
ChEBI: A derivative of tetracycline in which the amide function is substituted with a pyrrolidinomethyl group.
General Description
Rolitetracycline, N-(pyrrolidinomethyl)tetracycline(Syntetrin), was introduced for use by intramuscular or intravenousinjection. This derivative is made by condensingtetracycline with pyrrolidine and formaldehyde in the presenceof tert-butyl alcohol. It is very soluble in water (1 g dissolvesin about 1 mL) and provides a means of injecting theantibiotic in a small volume of solution. It has been recommendedfor cases when the oral dosage forms are not suitable,but it is no longer widely used.
Pharmaceutical Applications
2-N-pyrrolidinomethyl-tetracycline. A semisynthetic derivative
of tetracycline supplied as the nitrate sesquihydrate for
parenteral use.
It is not absorbed from the gastrointestinal tract. It is highly
soluble and therefore can be administered parenterally. Peak
plasma concentrations of 4–6 mg/L occur at 0.5–1 h after
350 mg intravenously. The plasma elimination half-life is 5–8 h.
About 50% of the dose is excreted in the urine, producing
high concentrations.
Intravenous administration is occasionally accompanied by
abnormal taste, shivering and rigors, hot flushes, facial reddening,
dizziness and, rarely, circulatory collapse. Symptoms
of myasthenia gravis have occasionally been exacerbated.
ROLITETRACYCLINE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | China | 9640 | 58 | Inquiry |
| InvivoChem | +1-708-310-1919 +1-13798911105 | United States | 6393 | 58 | Inquiry |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | China | 33350 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 52927 | 58 | Inquiry |
| RD International Technology Co., Limited | 18024082417 | China | 9268 | 58 | Inquiry |
| InvivoChem | 13549236410 | China | 6755 | 58 | Inquiry |
| Olix (Shanghai) Pharmaceutical Technology Co., Ltd | 17316404525 | China | 9721 | 58 | Inquiry |
| Wuhan Yingnuo Pharmaceutical Technology Co., Ltd. | +86-027-59232304 15387063101 | China | 9942 | 58 | Inquiry |
| Finetech Industry Limited | 027-87465837 19945049750 | China | 9649 | 58 | Inquiry |





