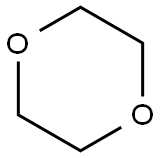
1,4-Dioxane
- CAS No.
- 123-91-1
- Chemical Name:
- 1,4-Dioxane
- Synonyms
- DIOX;Dioxan;P-DIOXANE;1,4-DIOXAN;4-Dioxane;1,4-Dioxacyclohexane;Dioxane-1,4;DIETHYLENE OXIDE;AAAAA;Pfu
- CBNumber:
- CB6240532
- Molecular Formula:
- C4H8O2
- Molecular Weight:
- 88.11
- MOL File:
- 123-91-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 11:33:24
| Melting point | 12 °C |
|---|---|
| Boiling point | 101 °C |
| Density | 1.034 g/mL at 25 °C(lit.) |
| vapor density | 3 (vs air) |
| vapor pressure | 27 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 54 °F |
| storage temp. | room temp |
| solubility | Soluble in acetone, alcohol, benzene, and ether (Weast, 1986). Miscible with most organic solvents (Huntress and Mulliken, 1941) including 2-methylpropanol, toluene, cychexanone, and cyclopentanone. |
| form | Solution |
| color | APHA: ≤20 |
| Odor | Mild ether-like odor detectable at 0.8 to 172 ppm (mean = 12 ppm) |
| PH | 6-8 (500g/l, H2O, 20℃) |
| explosive limit | 1.7-25.2%(V) |
| Water Solubility | SOLUBLE |
| Sensitive | Hygroscopic |
| λmax |
λ: 220 nm Amax: ≤0.70 λ: 235 nm Amax: ≤0.50 λ: 250 nm Amax: ≤0.20 λ: 270 nm Amax: ≤0.10 λ: 295-400 nm Amax: ≤0.01 |
| Merck | 14,3300 |
| BRN | 102551 |
| Henry's Law Constant | 4.89(x 10-6 atm?m3/mol) (static headspace-GC, Welke et al., 1998) |
| Exposure limits | TLV-TWA 25 ppm (≈90 mg/m3) (ACGIH), 100 ppm (MSHA and OSHA); carcinogenicity: Animal Sufficient Evidence (IARC). |
| Dielectric constant | 2.2(25℃) |
| Stability | Stable. Incompatible with oxidizing agents, oxygen, halogens, reducing agents, moisture. Highly flammable - note wide explosive range. May form explosive peroxides in storage (rate of formation increased by heating, evaporation or exposure to light). |
| InChIKey | RYHBNJHYFVUHQT-UHFFFAOYSA-N |
| LogP | -0.42 at 20℃ |
| CAS DataBase Reference | 123-91-1(CAS DataBase Reference) |
| IARC | 2B (Vol. 11, Sup 7, 71) 1999 |
| NIST Chemistry Reference | 1,4-Dioxane(123-91-1) |
| EPA Substance Registry System | 1,4-Dioxane (123-91-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H304-H315-H319-H335-H340-H350-H372-H412 | |||||||||
| Precautionary statements | P210-P273-P301+P310-P303+P361+P353-P305+P351+P338-P331 | |||||||||
| Hazard Codes | Xn,F,T | |||||||||
| Risk Statements | 45-46-11-36/38-48/23/24/25-65-66-40-36/37-19-41-37/38-39/23/24/25-23/24/25-48/20/22-38-22-36/37/38-10 | |||||||||
| Safety Statements | 9-16-36/37-46-45-53-7-62-26-24/25-23-S9-S46-S36/37-S16 | |||||||||
| OEL | Ceiling: 1 ppm (3.6 mg/m3) [30-minute] | |||||||||
| RIDADR | UN 1993 3/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | JG8225000 | |||||||||
| F | 8 | |||||||||
| Autoignition Temperature | 180 °C | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29329990 | |||||||||
| Toxicity | LD50 in mice, rats (ml/kg): 5.7, 5.2 orally (Laug) | |||||||||
| IDLA | 500 ppm | |||||||||
| NFPA 704 |
|
1,4-Dioxane price More Price(81)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | D201863 | 1,4-Dioxane ReagentPlus?, ≥99% | 123-91-1 | 500ML | ₹4784.65 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | RTC000093 | 1,4-Dioxane Pharmaceutical Secondary Standard; Certified Reference Material | 123-91-1 | 20ML | ₹12351.33 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1549 | 1,4-Dioxane Pharmaceutical Secondary Standard; Certified Reference Material | 123-91-1 | 3X1.2ML | ₹9103.83 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | D201863 | 1,4-Dioxane ReagentPlus?, ≥99% | 123-91-1 | 2.5L | ₹16497.3 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | D201863 | 1,4-Dioxane ReagentPlus?, ≥99% | 123-91-1 | 10L | ₹36534.38 | 2022-06-14 | Buy |
1,4-Dioxane Chemical Properties,Uses,Production
Description
1,4-dioxane is a clear liquid with ether-like odour. It is highly flammable and forms explosive peroxides in storage (rate of formation increased by heating, evaporation, or exposure to light). 1,4-Dioxane is incompatible with oxidising agents, oxygen, halogens, reducing agents, and moisture. Industrial applications of 1,4-dioxane are extensive, for instance, as solvent for cellulose acetate, ethyl cellulose, benzyl cellulose, resins, oils, waxes, and some dyes; as a solvent for paper, cotton, and textile processing; and for various organic and inorganic compounds and products. It is also used in automotive coolant liquid and in shampoos and other cosmetics as a degreasing agent and as a component of paint and varnish. Human exposures to 1,4-dioxane have been traced to multiple occupations and breathing of contaminated workplace air and drinking polluted water. Industrial uses of 1,4-dioxane are very many. For instance, it is used as solvent for celluloses, resins, lacquers, synthetic rubbers, adhesives, sealants, fats, oils, dyes, and protective coatings; as a stabiliser for chlorinated solvents and printing inks; and as a wetting and dispersing agent in textile processing agrochemicals and pharmaceuticals, in different processing of solvent-extraction processes, and in the preparation and manufacture of detergents.
Chemical Properties
1,4-Dioxane is a colorless, stable liquid with a faint, pleasant odor. Although it has been known as far back as 1863, it was not until 1929 that is became commercially available. It is chemically a di-ether obtained by the loss of water from two molecules of ethylene glycol. It is completely soluble in water, as well as most organic solvents. It is freely soluble in mineral, vegetable, blown and heat-bodied oils, and oil soluble dyes. Most waxes are more readily soluble in dioxane when heated and examples of these are beeswax, carnauba, montan, paraffin, gilsonite, and Japan wax.
Physical properties
Clear, colorless, very flammable, volatile liquid with a faint pleasant, ether-like odor. Experimentally determined detection and recognition odor threshold concentrations were 2.9 mg/m3 (800 ppbv) and 6.5 mg/m3 (1.8 ppmv), respectively (Hellman and Small, 1974).
Uses
1,4-Dioxane, the six-member cyclic diether, is used as an aluminum inhibitor in chlorinated solvents like 1,1,1-trichloroethane and as a solvent for certain resins and polymers.
Definition
ChEBI: A dioxane with oxygen atoms at positions 1 and 4.
General Description
A clear colorless liquid with a faint ethereal odor. Flash point 55°F. Slightly denser than water and soluble in water. Vapors heavier than air. Susceptible to autooxidation to form peroxides.
Air & Water Reactions
Highly flammable. When exposed to air 1,4-Dioxane undergoes autooxidation with formation of peroxides. In the distillation process peroxides will concentrate causing violent explosion. Water soluble.
Reactivity Profile
1,4-Dioxane is a flammable liquid; when exposed to air 1,4-Dioxane undergoes autooxidation with formation of peroxides. In the distillation process peroxides will concentrate causing violent explosion. The addition complex with sulfur trioxide (1:1) sometimes decomposes violently on storing at room temperature [Sisler, H. H. et al., Inorg. Synth., 1947, 2, p. 174]. Evaporation of boron trifluoride in aqueous 1,4-Dioxane with nitric acid led to an explosion upon addition of perchloric acid [MCA Guide, 1972, p. 312]. Explosive reaction with Raney nickel catalyst above 210° C {Mozingo R., Org. Synth., 1955, Coll. Vol. 3, p. 182].
Health Hazard
The toxicity of 1,4-dioxane is low in testanimals by all routes of exposure. However,in humans the toxicity of this compoundis severe. The target organs are theliver, kidneys, lungs, skin, and eyes. Exposureto its vapors as well as the absorptionthrough the skin or ingestion can cause poisoning,the symptoms of which include drowsiness,headache, respiratory distress, nausea,and vomiting. It causes depression of centralnervous system. There are reports of humandeaths from subacute and chronic exposures todioxane vapors at concentration levels rangingbetween 500 and 1000 ppm. Serious healthhazards may arise from its injurious effects onthe liver, kidneys, and brain. Rabbits died ofkidney injury resulting from repeated inhalationof 1,4-dioxane vapors for 30 days (Smyth1956). It is an irritant to the eyes, nose, skin,and lungs. In humans, a 1-minute exposure to5000-ppm vapors can cause lacrimation.
LC50 value, inhalation (rats): 13,000 ppm/2 h
LD50 value, oral (mice): 5700 mg/kg
1,4-Dioxane is an animal carcinogen oflow potential. Ingestion of high concentrationsof this compound at a level of7000–18,000 ppm in drinking water for14–23 months caused nasal and liver tumorsin rats (ACGIH 1986). Guinea pigs developedlung tumors.
Flammability and Explosibility
Dioxane is a highly flammable liquid (NFPA rating = 3). Its vapor is heavier than air and may travel a considerable distance to a source of ignition and flash back. Dioxane vapor forms explosive mixtures with air at concentrations of 2 to 22% (by volume). Fires involving dioxane should be extinguished with carbon dioxide or dry powder extinguishers.
Dioxane can form shock- and heat-sensitive peroxides that may explode on concentration by distillation or evaporation. Samples of this substance should always be tested for the presence of peroxides before distilling or allowing to evaporate. Dioxane should never be distilled to dryness.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenicdata. Poison by intraperitoneal route. Moderately toxic by ingestion and inhalation. Mildly toxic by skin contact. Human systemic effects by inhalation: lachrymation, conjunctiva irritation, convulsions, hgh blood pressure, unspecified respiratory and gastrointestinal system effects. Mutation data reported. An eye and slun irritant. The irritant effects probably provide sufficient warning, in acute exposures, to enable a worker to leave exposure before being seriously affected. Repeated exposure to low concentrations has resulted in human fatahties, the organs chefly affected being the liver and kidneys. A very dangerous fire and explosion hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Violent reaction with (H2 + Raney Ni), AgClO4. Can form dangerous peroxides when exposed to air. Potentially explosive reaction with nitric acid + perchloric acid, Raney nickel catalyst (above 210°C). Forms explosive mixtures with decaborane (impactsensitive), triethynylaluminum (sensitive to heating or drying). Violent reaction with sulfur trioxide. Incompatible with sulfur trioxide. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also GLYCOL ETHERS.
Carcinogenicity
1,4-Dioxane is reasonably anticipated to be a human carcinogen basedon sufficient evidence of carcinogenicity from studies in experimental animals.
storage
dioxane should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers. Containers of dioxane should be dated when opened and tested periodically for the presence of peroxides.
Incompatibilities
Dioxane can form potentially explosive peroxides upon long exposure to air. Dioxane may react violently with Raney nickel catalyst, nitric and perchloric acids, sulfur trioxide, and strong oxidizing reagents.
Waste Disposal
Excess dioxane and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
Precautions
Workers Should be careful during handling of 1,4-Dioxane and avoid open flames, sparks and smoking. Workers should wear proper protectives since 1,4-Dioxane in known as hazardous, cause damage to eyes, respiratory tract, liver and kidney.
1,4-Dioxane Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| V B Organics | +91-9912996647 +91-9912996647 | Telangana, India | 13 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| BASF India Limited | +91-2262785600 +91-2262785600 | Maharashtra, India | 209 | 58 | Inquiry |
| Mehk Chemicals Pvt Ltd | +91-9323157788 +91-8369781782 | Maharashtra, India | 32 | 58 | Inquiry |
| Kairav Chemofarbe Industries Ltd. KCIL | +91-222596836 +91-8879003729 | Maharashtra, India | 9 | 58 | Inquiry |
| Wellchem Laboratories | +91-9820795238 +91-9892737187 | Maharashtra, India | 30 | 58 | Inquiry |
| Sujay Chemicals | +91-2227700303 +91-9323420477 | Maharashtra, India | 7 | 58 | Inquiry |
| DeFINE CHEMICALS | +91-8010769889 +91-9320057099 | Maharashtra, India | 120 | 58 | Inquiry |
| Manas Petro Chem | +91-9004912487 +91-9004912487 | Maharashtra, India | 17 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ANJI BIOSCIENCES | 58 |
| V B Organics | 58 |
| JSK Chemicals | 58 |
| BASF India Limited | 58 |
| Mehk Chemicals Pvt Ltd | 58 |
| Kairav Chemofarbe Industries Ltd. KCIL | 58 |
| Wellchem Laboratories | 58 |
| Sujay Chemicals | 58 |
| DeFINE CHEMICALS | 58 |
| Manas Petro Chem | 58 |
Related articles
- Uses of 1,4-Dioxane
- 1,4-Dioxane was first identified in 1863 and became available for commercial use in the 1930s as a solvent in cellulose acetat....
- Jan 19,2022
- What is the application of 1,4-Dioxane?
- 1,4-Dioxane was used as a stabilizer for chlorinated organic solvents, beginning in the 1950s. Groundwater contamination has b....
- Oct 13,2021
- 1,4-Dioxane-Hazard and Toxicity
- 1,4-dioxane is a clear liquid with ether-like odour. It is highly flammable and forms explosive peroxides in storage (rate of ....
- Sep 5,2019
Related Qustion
- Q:How to synthesis 1,4-Dioxane?
- A:1,4-Dioxane, a cyclic ether first reported synthesized in 1863, poses a cancer risk when released into the air and people brea....
- Jul 2,2024





