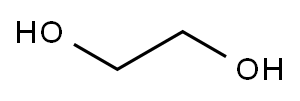
Ethylene glycol
- CAS No.
- 107-21-1
- Chemical Name:
- Ethylene glycol
- Synonyms
- Monoethylene glycol;dowtherm;1,2-Ethanediol;HOCH2CH2OH;2-Hydroxyethanol;Antifrogen N;Dihydroxyethane;Zerex;Glygen;Glykol
- CBNumber:
- CB7852707
- Molecular Formula:
- C2H6O2
- Molecular Weight:
- 62.07
- MOL File:
- 107-21-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/22 7:31:40
| Melting point | -13 °C (lit.) |
|---|---|
| Boiling point | 195-198 °C |
| Density | 1.113 g/mL at 25 °C (lit.) |
| vapor density | 2.1 (vs air) |
| vapor pressure | 0.08 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 230 °F |
| storage temp. | 2-8°C |
| solubility | water: miscible |
| form | Viscous Liquid |
| pka | 14.22(at 25℃) |
| color | blue |
| Odor | Odorless |
| Relative polarity | 0.79 |
| PH | 6-7.5 (100g/l, H2O, 20℃) |
| explosive limit | 3.2%(V) |
| Water Solubility | miscible |
| FreezingPoint | -11.5℃ |
| Sensitive | Hygroscopic |
| λmax |
λ: 260 nm Amax: ≤0.03 λ: 280 nm Amax: ≤0.01 |
| Merck | 14,3798 |
| BRN | 505945 |
| Exposure limits | Ceiling limit in air for vapor and mist 50 ppm (~125 mg/m3) (ACGIH); TWA 10 mg/m3 (particulates) (MSHA). |
| Dielectric constant | 37.0(20℃) |
| LogP | -1.36 at 25℃ |
| CAS DataBase Reference | 107-21-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,2-Ethanediol(107-21-1) |
| EPA Substance Registry System | Ethylene glycol (107-21-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H373 | |||||||||
| Precautionary statements | P260-P264-P270-P301+P312-P314-P501 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-36-41 | |||||||||
| Safety Statements | 26-39-36/37/39 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | KW2975000 | |||||||||
| Autoignition Temperature | 752 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29053100 | |||||||||
| Toxicity | LD50 in rats, guinea pigs (g/kg): 8.54, 6.61 orally (Smyth); in mice (ml/kg): 13.79 orally (Bornmann) | |||||||||
| NFPA 704 |
|
Ethylene glycol price More Price(64)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | PHR1046 | Ethylene glycol Pharmaceutical Secondary Standard; Certified Reference Material | 107-21-1 | 1G | ₹8250.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 900631 | Ethylene glycol anhydrous, ZerO2?, 99.8% | 107-21-1 | 2ML | ₹1645.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 85978 | Ethylene glycol analytical standard | 107-21-1 | 5ML | ₹15793.35 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 85978 | Ethylene glycol analytical standard | 107-21-1 | 10ML | ₹31038.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 721980 | Ethylene glycol solution NMR reference standard, 80% in DMSO-d6 (99.9 atom % D), NMR tube size 3?mm × 8?in. | 107-21-1 | 1PKG | ₹11210 | 2022-06-14 | Buy |
Ethylene glycol Chemical Properties,Uses,Production
Description
Ethylene glycol was first synthesized in 1859; however, it did not become a public health concern until after World War II. In fact, the first published series of deaths from ethylene glycol consumption involved 18 soldiers who drank antifreeze as a substitute for ethanol. Despite the early recognition that patients who drank ethanol in addition to ethylene glycol had prolonged survival when compared to those drinking ethylene glycol alone, antidotal treatment of ethylene glycol toxicity with ethanol was not evaluated until the 1960s. Today, ethylene glycol poisoning continues to be a public health problem, particularly in the southeastern United States. In 2009, US poison control centers received 5282 calls about possible ethylene glycol exposures, and the toxicology community believes these exposures are underreported.
Chemical Properties
Ethylene glycol,CH20HCH20H, also known as glycol,ethylene alcohol, glycol alcohol, and dihydric alcohol, is a colorless liquid. It is soluble in water and in alcohol. Ethyleneglycol has a low freezing point,-25°C (-13 OF), and is widely used as an antifreeze in automobiles and in hydraulic fluids. It is used as a solvent for nitrocellulose and in the manufacture of acrylonitrile, dynamites, and resins.
Uses
Ethylene glycol is used as an antifreeze inheating and cooling systems (e.g., automobileradiators and coolant for airplane motors).It is also used in the hydraulic brake fluids;as a solvent for paints, plastics, and inks; as a softening agent for cellophane; and in themanufacture of plasticizers, elastomers, alkydresins, and synthetic fibers and waxes.
Production Methods
Historically, ethylene glycol has been manufactured by hydrolyzing ethylene oxide. Presently, it is also produced commercially by oxidizing ethylene in the presence of acetic acid to form ethylene diacetate, which is hydrolyzed to the glycol, and acetic acid is recycled in the process .
Definition
ChEBI: A 1,2-glycol compound produced via reaction of ethylene oxide with water.
Preparation
Ethylene glycol is prepared by the hydration of ethylene oxide:

This reaction is carried out in a manner comparable to that described for the preparation of propylene glycol from propylene oxide . Ethylene glycol is a colourless liquid, b.p. 197??C.
Reactions
Glycol reacts (1) with sodium to form sodium glycol, CH2OH · CH2ONa, and disodium glycol, CH2ONa·CH2ONa; (2) with phosphorus pentachloride to form ethylene dichloride, CH2Cl·CH2Cl (3) with carboxy acids to form mono- and disubstituted esters, e.g., glycol monoacetate, CH2OH·CH2OOCCH3, glycol diacetate, CH3COOCH2 · CH2OOCCH3; (4) with nitric acid (with sulfuric acid), to form glycol mononitrate, CH2OH·CH2ONO2, glycol dinitrate, CH2ONO2 · CH2ONO2; (5) with hydrogen chloride, heated, to form glycol chlorohydrin (ethylene chlorohydrin, CH2OH·CHCl); (6) upon regulated oxidation to form glycollic aldehyde, CH2OH·CHO, glyoxal, CHO · CHO, glycollic acid, CH2OH·COOH, glyoxalic acid, CHO·COOH, oxalic acid, COOH·COOH.
General Description
Ethylene glycol is a clear, colorless syrupy liquid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Since Ethylene glycol is a liquid Ethylene glycol can easily penetrate the soil and contaminate groundwater and nearby streams.
Reactivity Profile
Mixing Ethylene glycol in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: chlorosulfonic acid, oleum, sulfuric acid, [NFPA 1991].
Hazard
Questionable carcinogen. Toxic by ingestion and inhalation. Lethal dose reported to be 100 cc.
Health Hazard
Inhalation of vapor is not hazardous. Ingestion causes stupor or coma, sometimes leading to fatal kidney injury.
Fire Hazard
Ethylene glycol is combustible.
Safety Profile
Human poison by ingestion. (Lethal dose for humans reported to be 100 mL.) Moderately toxic to humans by an unspecified route. Moderately toxic experimentally by ingestion, subcutaneous, intravenous, and intramuscular routes. Human systemic effects by ingestion and inhalation: eye lachrymation, general anesthesia, headache, cough, respiratory stimulation, nausea or vomiting, pulmonary, kidney, and liver changes. If ingested it causes initial central nervous system stimulation followed by depression. Later, it causes potentially lethal kidney damage. Very toxic in particulate form upon inhalation. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. A skin, eye, and mucous membrane irritant. Combustible when exposed to heat or flame; can react vigorously with oxidants. Moderate explosion hazard when exposed to flame. Iptes on contact with chromium trioxide, potassium permanganate, and sodium peroxide. Mixtures with ammonium dichromate, silver chlorate, sodium chlorite, and uranyl nitrate ipte when heated to 100°C. Can react violently with chlorosulfonic acid, oleum, H2SO4, HClO4, and Pass. Aqueous solutions may ignite silvered copper wires that have an applied D.C. voltage. To fight fire, use alcohol foam, water, foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
Ethylene glycol is used in antifreeze (especially as car radiator antifreeze) and in production of polyethylene terephthalate fibers and films; in hydraulic fluids; antifreeze and coolant mixtures for motor vehicles; electrolytic condensers; and heat exchangers. It is also used as a solvent and as a chemical intermediate for ethylene glycol dinitrate, glycol esters; resins, and for pharmaceuticals.
Environmental Fate
Ethylene glycol is considered an inert ingredient in pesticides. It
typically enters the environment through waste streams after
use of deicing products, where it is highly mobile in soil and
contaminates groundwater. Ethylene glycol is considered
‘readily biodegradable.’ It biodegrades relatively quickly; its
half-life (t1/2) is 2–12 days in soil.
Ethylene glycol is biodegraded in water under both aerobic
and anaerobic conditions within a day to a few weeks. In the
atmosphere, ethylene glycol photochemically degrades with
a t1/2 of approximately 2 days.
Solubility in organics
Miscible with water and alcohol, soluble in lower atifatic alcohols and ketones, Propylene glycol and Glycerin, poorly soluble in Hydrocarbons such as Terpenes as well as in Terpene alcohols, esters, etc.
Shipping
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required
Purification Methods
It is very hygroscopic, and also likely to contain higher diols. Dry it with CaO, CaSO4, MgSO4 or NaOH and distil it under vacuum. Dry further by reaction with sodium under nitrogen, reflux for several hours and distil. The distillate is then passed through a column of Linde type 4A molecular sieves and finally distil under nitrogen, from more molecular sieves. Then fractionally distil it. [Beilstein 1 IV 2369.]
Incompatibilities
Reacts with sulfuric acid, oleum, chlorosulfonic acid; strong oxidizing agents; strong bases; chromium trioxide; potassium permanganate; sodium peroxide. Hygroscopic (i.e., absorbs moisture from the air)
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Alternatively, ethylene glycol can be recovered from polyester plant wastes
Ethylene glycol Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Vardhaman P Golechha | 9704422000 | Telangana, India | 294 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Merck Ltd | +91-2262109800 +91-2262109000 | Maharashtra, India | 272 | 58 | Inquiry |
| Newage Industrial Oils Pvt Ltd NIOPL | +91-846005276 +91-8866035309 | Gujarat, India | 11 | 58 | Inquiry |
| Baron Organics | +91-9825029778 +91-9825029778 | Gujarat, India | 13 | 58 | Inquiry |
| Vinayak Organics Pvt Ltd | +91-9712940850 +91-9824010950 | Gujarat, India | 5 | 58 | Inquiry |
| Crystal India | +91-2226421194 +91-2226421194 | Maharashtra, India | 3 | 58 | Inquiry |
| MVL Medisynth Private Limited ( BALAJI AMINES LIMITED) | +91-8605000740 +91-9922472335 | Maharashtra, India | 9 | 58 | Inquiry |
| Reliance Industries Limited | +91-2235555000 +91-2235555000 | Maharashtra, India | 15 | 58 | Inquiry |
| Akry Organics Pvt Ltd | +91-9820361615 +91-9322211747 | Maharashtra, India | 3 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Vardhaman P Golechha | 58 |
| JSK Chemicals | 58 |
| Merck Ltd | 58 |
| Newage Industrial Oils Pvt Ltd NIOPL | 58 |
| Baron Organics | 58 |
| Vinayak Organics Pvt Ltd | 58 |
| Crystal India | 58 |
| MVL Medisynth Private Limited ( BALAJI AMINES LIMITED) | 58 |
| Reliance Industries Limited | 58 |
| Akry Organics Pvt Ltd | 58 |
Related articles
- Novel synthesis of Ethylene glycol: Nexus molecule outputs pure ethylene glycol
- The currently dominant approach to ethylene glycol synthesis is grounded in a two-step energy-intensive process that incorpora....
- Apr 16,2024
- Ethylene Glycol Poisoning
- Ethylene glycol poisoning is poisoning caused by drinking ethylene glycol. Early symptoms include intoxication, vomiting and a....
- Nov 17,2022
- The application of ethylene glycol
- Ethylene glycol is also known as "ethylene glycol", "1,2-ethylene glycol", or EG for short.
- Mar 18,2022
107-21-1(Ethylene glycol)Related Search:
1of4
chevron_right




