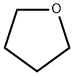
Tetrahydrofuran
- CAS No.
- 109-99-9
- Chemical Name:
- Tetrahydrofuran
- Synonyms
- THF;Oxolane;PTMEG 2000;PTHF;Butylene oxide;Tetrahydrofurane;oxolan;PTMEG 1000;1,4-Epoxybutane;TETRAMETHYLENE ETHER GLYCOL 2000 POLYMER
- CBNumber:
- CB6852795
- Molecular Formula:
- C4H8O
- Molecular Weight:
- 72.11
- MOL File:
- 109-99-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/4/9 17:45:08
| Melting point | -108°C |
|---|---|
| Boiling point | 66 °C |
| Density | 0.887 g/mL at 20 °C |
| vapor density | 2.5 (vs air) |
| vapor pressure | <0.01 mm Hg ( 25 °C) |
| refractive index |
n |
| Flash point | >230 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | water: soluble |
| form | Liquid |
| Specific Gravity | 0.89 |
| color | <10(APHA) |
| Odor | Ethereal, detectable at 2 to 50 ppm |
| PH Range | 7 |
| Relative polarity | 0.207 |
| PH | 7-8 (200g/l, H2O, 20℃) |
| explosive limit | 1.5-12.4%(V) |
| Water Solubility | miscible |
| FreezingPoint | -108℃ |
| Sensitive | Air Sensitive & Hygroscopic |
| λmax |
λ: 245 nm Amax: ≤0.26 λ: 275 nm Amax: ≤0.046 λ: 315 μm Amax: ≤0.0044 |
| Merck | 14,9211 |
| BRN | 102391 |
| Henry's Law Constant | 1.54 (static headspace-GC, Welke et al., 1998) |
| Exposure limits | TLV-TWA 200 ppm (590 mg/m3) (ACGIH, MSHA, and OSHA); STEL 250 ppm (ACGIH); IDLH 20,000 ppm (NIOSH). |
| Dielectric constant | 11.6(-70℃) |
| Stability | Stable. Incompatible with halogens, strong oxidizing agents, strong reducing agents, strong bases, oxygen. May generate explosive peroxides in storage if in contact with air. Highly flammable. Store at room temperature under nitrogen. Hazardous polymerisation may occur. Light sensitive. May contain 2,6-di-tertbutyl-4-methylphenol (BHT) as a s |
| InChIKey | WYURNTSHIVDZCO-UHFFFAOYSA-N |
| LogP | 0.45 at 25℃ |
| Surface tension | 27.31mN/m at 293.15K |
| CAS DataBase Reference | 109-99-9(CAS DataBase Reference) |
| IARC | 2B (Vol. 119) 2019 |
| NIST Chemistry Reference | Furan, tetrahydro-(109-99-9) |
| EPA Substance Registry System | Tetrahydrofuran (109-99-9) |
| Absorption |
≤0.0044 at 315nm ≤0.005 at 350nm ≤0.005 at 400nm ≤0.02 at 300nm ≤0.046 at 275nm ≤0.18 at 250nm ≤0.26 at 245nm ≤1.0 at 212nm |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H302-H319-H335-H336-H351 | |||||||||
| Precautionary statements | P201-P202-P210-P301+P312-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | Xi,F,Xn | |||||||||
| Risk Statements | 36/37/38-36/37-19-11-40 | |||||||||
| Safety Statements | 26-36-33-29-16-46-37-13 | |||||||||
| RIDADR | UN 2924 3/PG 2 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 200 ppm (590 mg/m3), STEL: 250 ppm (735 mg/m3) | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | MD0916000 | |||||||||
| F | 3-10-23 | |||||||||
| Autoignition Temperature | 610 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29321100 | |||||||||
| Hazardous Substances Data | 109-99-9(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 oral (rat) 2880 mg/kg LC50 inhal (rat) 21,000 ppm (3 h) PEL (OSHA) 200 ppm (590 mg/m3) TLV-TWA (ACGIH) 200 ppm (590 mg/m3) STEL (ACGIH) 250 ppm (737 mg/m3) |
|||||||||
| IDLA | 2,000 ppm [10% LEL] | |||||||||
| NFPA 704 |
|
Tetrahydrofuran price More Price(101)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | RTC000088 | Tetrahydrofuran Pharmaceutical Secondary Standard; Certified Reference Material | 109-99-9 | 20ML | ₹12351.33 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1561 | Tetrahydrofuran Pharmaceutical Secondary Standard; Certified Reference Material | 109-99-9 | 3X1.2ML | ₹9103.83 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 900636 | Tetrahydrofuran anhydrous, inhibitor-free, ZerO2?, ≥99.9% | 109-99-9 | 4X2ML | ₹1970.15 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 87369 | Tetrahydrofuran Selectophore?, ≥99.5% | 109-99-9 | 100ML | ₹4990.33 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 87369 | Tetrahydrofuran Selectophore?, ≥99.5% | 109-99-9 | 500ML | ₹15588 | 2022-06-14 | Buy |
Tetrahydrofuran Chemical Properties,Uses,Production
Chemical Properties
Tetrahydrofuran (THF) is an industrial solvent widely recognized for its unique combination of useful properties. DuPont THF is better than 99.9% pure with a small (0.025-0.040 wt % ) amount of butylated hydroxytoluene (BHT, 4-methyl-2,6-di-tertbutyl phenol) added as an antioxidant. Tetrahydrofuran is a cycloaliphatic ether and is not "photochemically reactive" as defined in Section k of Los Angeles County's Rule 66 (equivalent to Rule 442 of the Southern California Air Pollution Control District). THF has an ethereal odor. The Odor Threshold is listed @ 3.8 (3M), 20-50ppm, and 31ppm. It is also a common laboratory reagent and an intermediate in chemical syntheses of consumer and industrial products such as nutritionals, pharmaceuticals, and insecticides (HSDB, 2011).
Uses
Tetrahydrofuran is used primarily (80%) to make polytetramethylene ether glycol, the base polymer used primarily in the manufacture of elastomeric fibers (e.g., spandex) as well as polyurethane and polyester elastomers (e.g., artificial leather, skateboard wheels). The remainder (20%) is used in solvent applications (e.g., pipe cements, adhesives, printing inks, and magnetic tape) and as a reaction solvent in chemical and pharmaceutical syntheses.
Uses
Butylene oxide is used as a fumigant and inadmixture with other compounds. It is usedto stabilize fuel with respect to color andsludge formation.
General Description
A clear colorless liquid with an ethereal odor. Less dense than water.Flash point 6°F. Vapors are heavier than air.
Reactivity Profile
Tetrahydrofuran reacts violently with oxidizing agents leading to fires and explosions [Handling Chemicals Safely 1980. p. 891]. Subject to peroxidation in the air. Peroxides or their products react exothermically with lithium aluminum hydride [MCA Guide for Safety 1973]. Thus, use as a solvent for lithium aluminum hydride has led to fires. Using potassium hydroxide or sodium hydroxide to dry impure Tetrahydrofuran that contains peroxides has resulted in explosions. A violent explosion occurred during the preparation of sodium aluminum hydride from sodium and aluminum in a medium of Tetrahydrofuran [Chem. Eng. News 39(40):57. 1961]. THF forms explosive products with 2-aminophenol [Lewis 3227].
Flammability and Explosibility
THF is extremely flammable (NFPA rating = 3), and its vapor can travel a
considerable distance to an ignition source and "flash back." A 5% solution of THF
in water is flammable. THF vapor forms explosive mixtures with air at
concentrations of 2 to 12% (by volume). Carbon dioxide or dry chemical
extinguishers should be used for THF fires.
THF can form shock- and heat-sensitive peroxides, which may explode on
concentration by distillation or evaporation. Always test samples of THF for the
presence of peroxides before distilling or allowing to evaporate. THF should never
be distilled to dryness.
Potential Exposure
The primary use of tetrahydrofuran is as a solvent to dissolve synthetic resins, particularly polyvinyl chloride and vinylidene chloride copolymers. It is also used to cast polyvinyl chloride films, to coat substrates with vinyl and vinylidene chloride; and to solubilize adhesives based on or containing polyvinyl chloride resins. A second large market for THF is as an electrolytic solvent in the Grignard reaction-based production of tetramethyl lead. THF is used as an intermediate in the production of polytetramethylene glycol.
Carcinogenicity
THF showed little evidence of mutagenic activity in a variety of in vitro and in vivo assays.
storage
THF should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers. Containers of THF should be dated when opened and tested periodically for the presence of peroxides.
Incompatibilities
Forms thermally explosive peroxides in air on standing (in absence of inhibitors). Peroxides can be detonated by heating, friction, or impact. Reacts violently with strong oxidizers, strong bases and some metal halides. Attacks some forms of plastics, rubber and coatings.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Concentrated waste containing peroxides-perforation of a container of the waste from a safe distance followed by open burning.
Tetrahydrofuran Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 429 | 58 | Inquiry |
| ULTIMA CHEMICALS | +91 7208029458 | Mumbai, India | 95 | 58 | Inquiry |
| Evans Fine Chem | +91-9821340302 +91-9821340302 | Maharashtra, India | 286 | 58 | Inquiry |
| Azeocryst Organic Private Limited | +91-8879077780 +91-8879077780 | Maharashtra, India | 32 | 58 | Inquiry |
| BASF India Limited | +91-2262785600 +91-2262785600 | Maharashtra, India | 209 | 58 | Inquiry |
| Dhara Industries | +91-9322395199 +91-9322395199 | Mumbai, India | 190 | 58 | Inquiry |
| Globe Chemie | +91-9130055886 +91-9373912785 | Maharashtra, India | 2 | 58 | Inquiry |
| Suparna Chemicals Ltd | +91-2222027446 +91-9820623254 | Maharashtra, India | 19 | 58 | Inquiry |
| Merck Ltd | +91-2262109800 +91-2262109000 | Maharashtra, India | 272 | 58 | Inquiry |
| Kairav Chemofarbe Industries Ltd. KCIL | +91-222596836 +91-8879003729 | Maharashtra, India | 9 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ANJI BIOSCIENCES | 58 |
| ULTIMA CHEMICALS | 58 |
| Evans Fine Chem | 58 |
| Azeocryst Organic Private Limited | 58 |
| BASF India Limited | 58 |
| Dhara Industries | 58 |
| Globe Chemie | 58 |
| Suparna Chemicals Ltd | 58 |
| Merck Ltd | 58 |
| Kairav Chemofarbe Industries Ltd. KCIL | 58 |
Related articles
- A brief description of Tetrahydrofuran
- Tetrahydrofuran is a colorless and transparent liquid with an ether-like odor. It is a volatile liquid having a boiling point ....
- Mar 11,2024
- A moderate polar aprotic solvent-Tetrahydrofuran
- Tetrahydrofuran is a moderate polar aprotic solvent. THF has a moderate polarity thanks to the electronegativity difference be....
- Jan 4,2024
- Chemical Reactivity of Tetrahydrofuran
- Tetrahydrofuran(THF) is a colorless, volatile, flammable, pleasant-smelling liquid with low viscosity and a bp of 64°C. It is ....
- Jan 25,2022
109-99-9(Tetrahydrofuran)Related Search:
1of4
chevron_right




