Zinc tetrafluoroborate
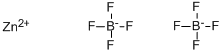
- CAS No.
- 13826-88-5
- Chemical Name:
- Zinc tetrafluoroborate
- Synonyms
- ZINC FLUOBORATE;ZINCFLUOROBOTATE;ZINC FLUOROBORATE;ZINC BOROFLUORIDE;zinc(ii)fluoborate;ZINC TETRAFLUOBORATE;ZINC TETRAFLUOROBORATE;Zinc fluoborate hydrate;tetrafluoro-borate(1-zinc;Zinkbis(tetrafluoroborat)
- CBNumber:
- CB0490982
- Molecular Formula:
- B2F8Zn
- Molecular Weight:
- 239
- MOL File:
- 13826-88-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/4 15:16:07
| Density | 1.43 |
|---|---|
| form | crystal |
| color | white |
| CAS DataBase Reference | 13826-88-5(CAS DataBase Reference) |
| EPA Substance Registry System | Borate(1-), tetrafluoro-, zinc (2:1) (13826-88-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS05 |
|---|---|
| Signal word | Danger |
| Hazard statements | H314-H302+H312+H332 |
| Precautionary statements | P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501 |
| Hazard Codes | C |
| Risk Statements | 34 |
| Safety Statements | 45-36/37/39-26 |
| HS Code | 28269080 |
Zinc tetrafluoroborate Chemical Properties,Uses,Production
Chemical Properties
clear colorless solution
Uses
Plating and bonderizing, resin curing.
General Description
Colorless odorless aqueous solution. Sinks and mixes with water.
Reactivity Profile
Acidic inorganic salts, such as Zinc tetrafluoroborate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
Ingestion may cause irritation or corrosion of the alimentary tract. Contact with eyes or skin causes irritation.
Zinc tetrafluoroborate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jayfluoride PVT LTD | +91-8128987973 +91-6356977002 | Gujarat, India | 27 | 58 | Inquiry |
| P J Chemicals | 08069013893Ext 828 | Gujarat, India | 29 | 58 | Inquiry |
| Subala Labchem | 08046070315 | Delhi, India | 23 | 58 | Inquiry |
| Jay Intermediates & Chemicals | 08048956835 | Gujarat, India | 21 | 58 | Inquiry |
| Arihant Unique Ventures Private Limited | 08068442271Ext 490 | Mumbai, India | 1 | 58 | Inquiry |
| Arnav Agencies | 08048371986Ext 586 | Mumbai, India | 1 | 58 | Inquiry |
| Aspire Speciality Chemicals | 08128987973 | Gujarat, India | 13 | 58 | Inquiry |
| Hindustan Chemical Corporation | 08046059377 | Gujarat, India | 29 | 58 | Inquiry |
| Mahavir Enterprises | 08048372566Ext 832 | Delhi, India | 3 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21669 | 55 | Inquiry |





