Fluoroboric acid
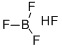
- CAS No.
- 16872-11-0
- Chemical Name:
- Fluoroboric acid
- Synonyms
- TETRAFLUOROBORIC ACID;FLUOBORIC ACID;HYDROFLUOROBORIC ACID;HYDROGEN TETRAFLUOROBORATE;tetrafluoroboric acid solution;48 en tetrafL;fluoroboric;borofluorid;Borofiuoric acid
- CBNumber:
- CB6853202
- Molecular Formula:
- BF3.FH
- Molecular Weight:
- 87.81
- MOL File:
- 16872-11-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/21 16:43:18
| Melting point | -90°C |
|---|---|
| Boiling point | 130°C (dec.) |
| Density | 1.38 g/mL at 20 °C(lit.) |
| vapor density | 3 (vs air) |
| vapor pressure | 5 mmHg ( 20 °C) |
| refractive index | nD20 of a 20% aq soln 1.3284 |
| Flash point | −40 °F |
| solubility | very soluble in H2O, ethanol |
| pka | -4.9[at 20 ℃] |
| form | Liquid |
| color | Light Yellow |
| Odor | Odorless |
| Water Solubility | MISCIBLE |
| Merck | 14,4149 |
| Exposure limits |
ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3 |
| Stability | Stable. Incompatible with strong bases, cyanides. |
| CAS DataBase Reference | 16872-11-0(CAS DataBase Reference) |
| EPA Substance Registry System | Fluoroboric acid (16872-11-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314 | |||||||||
| Precautionary statements | P280-P301+P330+P331-P303+P361+P353-P305+P351+P338+P310 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 34 | |||||||||
| Safety Statements | 26-36/37/39-45-37-27 | |||||||||
| RIDADR | UN 1775 8/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | ED2685000 | |||||||||
| F | 3 | |||||||||
| Hazard Note | Corrosive | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28111990 | |||||||||
| NFPA 704 |
|
Fluoroboric acid price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 207934 | Tetrafluoroboric acid solution 48?wt. % in H2O | 16872-11-0 | 25G | ₹1980.98 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 207934 | Tetrafluoroboric acid solution 48?wt. % in H2O | 16872-11-0 | 500G | ₹3799.58 | 2022-06-14 | Buy |
| ALFA India | ALF-L14037-36 | Tetrafluoroboric acid, ca 50% w/w aq. soln. | 16872-11-0 | 500g | ₹3074 | 2022-05-26 | Buy |
| ALFA India | ALF-L14037-22 | Tetrafluoroboric acid, ca 50% w/w aq. soln. | 16872-11-0 | 100?g | ₹1979 | 2022-05-26 | Buy |
| ALFA India | ALF-L14037-0E | Tetrafluoroboric acid, ca 50% w/w aq. soln. | 16872-11-0 | 2500g | ₹15306 | 2022-05-26 | Buy |
Fluoroboric acid Chemical Properties,Uses,Production
Chemical Properties
Fluoboric acid is a colourless liquid, completely solvable in water. Fluoboric acid is a stable chemical substance and extremely reactive or incompatible with strong bases, cyanides. It is very corrosive in the presence of steel, aluminium, zinc, and copper and highly corrosive in the presence of glass and stainless steel.
Uses
Tetrafluoroboric acid is used in conjunction with various methods of cleaning and treat the surface of substrates. It is used as radiotracers.
General Description
A colorless odorless poisonous liquid. Boiling point 130°C. Corrosive to metals and tissue. Fluoroboric acid is used in electroplating, metal cleaning and making diazo salts.
Air & Water Reactions
Soluble in water with release of heat.
Reactivity Profile
Fluoroboric acid is a strong acid. Reacts exothermically with chemical bases (examples: amines, amides, and inorganic hydroxides). These reactions can generate dangerously large amounts of heat in small spaces. Dissolution in water or the dilution of a concentrated aqueous solution may generate significant heat. Reacts with active metals, including such structural metals as aluminum and iron, to release hydrogen, a flammable gas. Can initiate the polymerization of certain alkenes. Reacts with cyanide compounds to release gaseous hydrogen cyanide. Generates flammable and/or toxic gases in contact with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and strong reducing agents. Additional gas-generating reactions occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and carbonates. May catalyze (increase the rate of) chemical reactions. Attempted drying of the acid with acetic anhydride caused an explosion at 0°C [J. Organomet. Chem., 1975, 94, 319].
Hazard
Highly toxic, corrosive, irritant.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
A corrosive acid. When heated to decomposition it emits toxic vapors of B and F-.
Potential Exposure
Used as a catalyst for acetal synthesis and cellulose esters; a metal surface cleaning agent; an alu minum electrolytic finishing agent; a stripping solution for the removal of solder and plated metals; and an intermedi ate in making fluoroborate salt.
Shipping
UN1775 Fluoroboric acid, Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
Crystallise fluoroboric acid several times from conductivity water. It can be stored in a glass vessel at room temperature. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 221-222 1963.]
Incompatibilities
A strong acid. Reacts violently with chemically active metals; strong bases, releasing flammable hydrogen gas.
Fluoroboric acid Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| S B Chemicals | +91-9979887375 +91-9825146049 | Gujarat, India | 13 | 58 | Inquiry |
| Jayfluoride PVT LTD | +91-8128987973 +91-6356977002 | Gujarat, India | 27 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Scientific OEM | +91-22- 2343 7546 / 2341 3094 | New Delhi, India | 1996 | 38 | Inquiry |
| ALPHA CHEMIKA | +91-22-22061123 +91-22-66382501 | Mumbai, India | 1681 | 43 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| LOBA CHEMIE PVT.LTD. | 91-22-6663 6699 | Mumbai, India | 3077 | 58 | Inquiry |
| Jay Intermediates & Chemicals | 08048956835 | Gujarat, India | 21 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| JSK Chemicals | 58 |
| S B Chemicals | 58 |
| Jayfluoride PVT LTD | 58 |
| Alfa Aesar | 58 |
| Scientific OEM | 38 |
| ALPHA CHEMIKA | 43 |
| Central Drug House(P) Ltd. | 58 |
| Triveni chemicals | 58 |
| LOBA CHEMIE PVT.LTD. | 58 |
| Jay Intermediates & Chemicals | 58 |
Related articles
- How to synthesize Fluoroboric acid?
- Fluoroboric acid is an inorganic, corrosive, strong acid with the chemical formula [H+][BF4-]. Available in various quantities....
- May 21,2024
- Fluoroboric acid: properties, applications and safety
- Fluoroboric acid is a strong Lewis acid, forms stable complexes, used in dental implants and PZT etching. However, corrosive a....
- Jul 31,2023
- Fluoroboric acid-Uses in Organic Analysis
- Fluoroboric acid or tetrafluoroboric acid (archaically, fluoboric acid) is an inorganic compound with the chemical formula [H+....
- Jan 10,2020
16872-11-0(Fluoroboric acid)Related Search:
1of4
chevron_right




