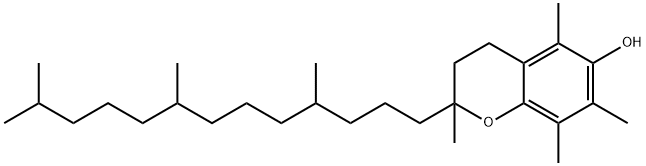
DL-α-Tocopherol
- CAS No.
- 10191-41-0
- Chemical Name:
- DL-α-Tocopherol
- Synonyms
- A-TOCOPHEROL;DL-ALPHA-TOCOPHEROL;DL-TOCOPHEROL;RAC-ALPHA-TOCOPHEROL;ALL-RAC-ALPHA-TOCOPHEROL;all-rac-α-Tocopherol;DL-ALPHA TOCOPHERYL ACETATE 500;(R)-2,5,7,8-tetraMethyl-2-((4R,8R)-4,8,12-triMethyltridecyl)chroMan-6-ol;3,;IRGANOX E 201
- CBNumber:
- CB5275357
- Molecular Formula:
- C29H50O2
- Molecular Weight:
- 430.71
- MOL File:
- 10191-41-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/2/13 18:31:58
| Melting point | 2-4°C |
|---|---|
| alpha | [α]D20 - 0.02 - +0.02゜ (neat) |
| Boiling point | 200-220°C 0,1mm |
| Density | 0.950 g/mL at 20 °C(lit.) |
| vapor pressure | <1 hPa ( 20 °C) |
| refractive index |
n |
| Flash point | 240°C |
| storage temp. | 2-8°C |
| solubility | H2O: insoluble |
| form | Pale yellow oil |
| pka | 11.40±0.40(Predicted) |
| color | Colourless to Dark Yellow |
| PH | 7 (20°C, 50g/L in H2O, slurry) |
| Odor | at 100.00?%. bland |
| biological source | synthetic |
| Water Solubility | Miscible with chloroform, vegetable oils, ether, acetone and alcohol. Immiscible with water. |
| Sensitive | Light Sensitive |
| Merck | 14,9495 |
| BRN | 94012 |
| InChIKey | GVJHHUAWPYXKBD-IEOSBIPESA-N |
| LogP | 12.2 at 25℃ |
| CAS DataBase Reference | 10191-41-0(CAS DataBase Reference) |
| EPA Substance Registry System | 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)- (10191-41-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H317 | |||||||||
| Precautionary statements | P261-P272-P280-P333+P313-P362+P364-P501 | |||||||||
| Hazard Codes | Xi,T,F | |||||||||
| Risk Statements | 36/37/38-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 26-37/39-45-36/37-16-7 | |||||||||
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | GA8746000 | |||||||||
| F | 8-10-23 | |||||||||
| Autoignition Temperature | 280°C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29362800 | |||||||||
| Hazardous Substances Data | 10191-41-0(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
DL-α-Tocopherol price More Price(18)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | T3251 | (±)-α-Tocopherol synthetic, ≥96% (HPLC) | 10191-41-0 | 5G | ₹1959.33 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T3251 | (±)-α-Tocopherol synthetic, ≥96% (HPLC) | 10191-41-0 | 25G | ₹4026.9 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | V-020 | (±)−α−Tocopherol (Vitamin E) solution 1.0?mg/mL in methanol, ampule of 1?mL, certified reference material, Cerilliant? | 10191-41-0 | 1ML | ₹8032.15 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T3251 | (±)-α-Tocopherol synthetic, ≥96% (HPLC) | 10191-41-0 | 100G | ₹10229.63 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T3251 | (±)-α-Tocopherol synthetic, ≥96% (HPLC) | 10191-41-0 | 500G | ₹25124.83 | 2022-06-14 | Buy |
DL-α-Tocopherol Chemical Properties,Uses,Production
Chemical Properties
1mg = 1.1 IU
Uses
dl-α-Tocopherol is the racemic analog of α-Tocopherol (T526125), the most bioactive of the naturally occurring forms of Vitamin E. Richest sources are green vegetables, grains, and oils, particularly palm, safflower and sunflower oils. dl-α-Tocopherol is an antioxidant that protects cell membrane lipids from oxidative damage.
Production Methods
Naturally occurring tocopherols are obtained by the extraction or molecular distillation of steam distillates of vegetable oils; for example, alpha tocopherol occurs in concentrations of 0.1–0.3% in corn, rapeseed, soybean, sunflower, and wheat germ oils.Beta and gamma tocopherol are usually found in natural sources along with alpha tocopherol. Racemic synthetic tocopherols may be prepared by the condensation of the appropriate methylated hydroquinone with racemic isophytol.
General Description
TGF-β3 (transforming growth factor-β3) belongs to the TGF β superfamily. The TGFβ3 gene is mapped to human chromosome 14q24.3
Pharmaceutical Applications
Alpha tocopherol is primarily recognized as a source of vitamin E,
and the commercially available materials and specifications reflect
this purpose. While alpha tocopherol also exhibits antioxidant
properties, the beta, delta, and gamma tocopherols are considered
to be more effective as antioxidants.
Alpha-tocopherol is a highly lipophilic compound, and is an
excellent solvent for many poorly soluble drugs.Of widespread
regulatory acceptability, tocopherols are of value in oil- or fat-based
pharmaceutical products and are normally used in the concentration
range 0.001–0.05% v/v. There is frequently an optimum
concentration; thus the autoxidation of linoleic acid and methyl
linolenate is reduced at low concentrations of alpha tocopherol, and
is accelerated by higher concentrations. Antioxidant effectiveness
can be increased by the addition of oil-soluble synergists such as
lecithin and ascorbyl palmitate.
Alpha tocopherol may be used as an efficient plasticizer. It has
been used in the development of deformable liposomes as topical
formulations.
d-Alpha-tocopherol has also been used as a non-ionic surfactant
in oral and injectable formulations.
Safety
Tocopherols (vitamin E) occur in many food substances that are
consumed as part of the normal diet. The daily nutritional
requirement has not been clearly defined but is estimated to be
3.0–20.0 mg. Absorption from the gastrointestinal tract is dependent
upon normal pancreatic function and the presence of bile.
Tocopherols are widely distributed throughout the body, with some
ingested tocopherol metabolized in the liver; excretion of metabolites
is via the urine or bile. Individuals with vitamin E deficiency are
usually treated by oral administration of tocopherols, although
intramuscular and intravenous administration may sometimes be
used.
Tocopherols are well tolerated, although excessive oral intake
may cause headache, fatigue, weakness, digestive disturbance, and
nausea. Prolonged and intensive skin contact may lead to erythema
and contact dermatitis.
The use of tocopherols as antioxidants in pharmaceuticals and
food products is unlikely to pose any hazard to human health since
the daily intake from such uses is small compared with the intake of
naturally occurring tocopherols in the diet.
The WHO has set an acceptable daily intake of tocopherol used
as an antioxidant at 0.15–2.0 mg/kg body-weight.
storage
Tocopherols are oxidized slowly by atmospheric oxygen and
rapidly by ferric and silver salts. Oxidation products include
tocopheroxide, tocopherylquinone, and tocopherylhydroquinone,
as well as dimers and trimers. Tocopherol esters are more stable to
oxidation than the free tocopherols but are in consequence less
effective antioxidants.
Tocopherols should be stored under an inert gas, in an airtight
container in a cool, dry place and protected from light.
Purification Methods
Dissolve dl--tocopherol in anhydrous MeOH (15mL/g) cool to -6o for 1hour, then chill in a Dry-ice/acetone bath; crystallisation is induced by scratching with a glass rod. The dl--acetate [52225-20-4] (see DL-vitamin E actetate below) is a viscous yellow liquid with m -7o, b 184o/0.01mm, 224o/0.3mm, d 4 20 0.953, n D 20 1.496. It is used as a standard for Vitamin E activity where the unit of activity is attained with 1mg of pure dl--acetate. [Friedrich “Vitamins” Water de Guyter Publ, Berlin 1988, Beilstein 17/4 V 168.]
Incompatibilities
Tocopherols are incompatible with peroxides and metal ions, especially iron, copper, and silver. Tocopherols may be absorbed into plastic.
Regulatory Status
GRAS listed. Accepted in Europe as a food additive. Included in the FDA Inactive Ingredients Database (IV injections, powder, lyophilized powder for liposomal suspension; oral capsules, tablets, and topical preparations). Included in the Canadian List of Acceptable Non-medicinal Ingredients. Included in nonparenteral medicines licensed in the UK.
DL-α-Tocopherol Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Shreenath Chemicals | +91-8369634120 +91-8369634120 | Maharashtra, India | 48 | 58 | Inquiry |
| Unicorn Petroleum Industries Private Limited | +91-2225299990 +91-9930731710 | Maharashtra, India | 22 | 58 | Inquiry |
| Merck Ltd | +91-2262109800 +91-2262109000 | Maharashtra, India | 272 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5870 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6905 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Purenso Global | 08047632195 | Madhya Pradesh, India | 44 | 58 | Inquiry |
| Nutrolics International | 08047305173 | Delhi, India | 8 | 58 | Inquiry |
| Avirahi Pharmaceuticals LLP | 08046055270 | Mumbai, India | 3 | 58 | Inquiry |
| Triveni Interchem Private Limited (Group Of Triveni... | 08046042087 | Gujarat, India | 497 | 58 | Inquiry |
Related articles
- Synthesis and application of α-Tocopherol
- Vitamin E exists in eight different forms, four tocopherols and four tocotrienols. All feature a chromane ring, with a hydroxy....
- Nov 30,2021





