Fluorine
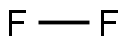
- CAS No.
- 7782-41-4
- Chemical Name:
- Fluorine
- Synonyms
- F2;Fluoro;Fluor;fluorine gas;THRB;C00742;FLUORINE;difluorine;Bifluoriden;Fluoruri acidi
- CBNumber:
- CB1221811
- Molecular Formula:
- F2
- Molecular Weight:
- 38
- MOL File:
- 7782-41-4.mol
- Modify Date:
- 2024/3/14 15:18:25
| Melting point | -220°C |
|---|---|
| Boiling point | -188°C |
| Density | 1.695(15℃) |
| vapor pressure | >760 mmHg at 20 °C |
| refractive index | 1.000195 |
| storage temp. | -20°C |
| solubility | reacts with H2O |
| form | pale yellow gas |
| color | pale |
| Odor | Strong ozone-like odor detectable at 0.1 to 0.2 ppm |
| Water Solubility | reacts |
| Exposure limits | TLV-TWA 1 ppm (~2 mg/m3) (ACGIH and MSHA), 0.1 ppm (OSHA); IDLH 25 ppm (NIOSH). |
| Dielectric constant | 1.5(-201℃) |
| Stability | Stable. Extremely strong oxidant which may react violently with combustible materials, including plastics, reducing agents and organic material. Reacts with water to form corrosive acids. |
| CAS DataBase Reference | 7782-41-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Fluorine(7782-41-4) |
| EPA Substance Registry System | Fluorine (7782-41-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS04,GHS03,GHS06,GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H270-H314-H330 | |||||||||
| Precautionary statements | P220-P244-P370+P376-P403-P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501 | |||||||||
| Hazard Codes | T+,C | |||||||||
| Risk Statements | 7-26-35 | |||||||||
| Safety Statements | 9-26-36/37/39-45 | |||||||||
| RIDADR | UN 1045/9192 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.1 ppm (0.2 mg/m3) | |||||||||
| DOT Classification | 2.3, Hazard Zone A (Gas poisonous by inhalation) | |||||||||
| HazardClass | 2.3 | |||||||||
| Toxicity | LC50 (1 hr) inhalation by rats, mice, guinea pigs: 185, 150, 170 ppm (by vol) (Keplinger, Suissa) | |||||||||
| IDLA | 25 ppm | |||||||||
| NFPA 704 |
|
Fluorine price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | SAB4500821 | Anti-THRB (AP2, Cleaved-Arg327) antibody produced in rabbit affinity isolated antibody | 7782-41-4 | 100μG | ₹50560.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | SAB4500820 | Anti-THRB antibody produced in rabbit affinity isolated antibody | 7782-41-4 | 100μG | ₹50560.5 | 2022-06-14 | Buy |
Fluorine Chemical Properties,Uses,Production
Description
Fluorine is a highly toxic, pale yellow gas about 1.3 times as heavy as air at atmospheric temperature and pressure. When cooled below its boiling point (-306.8°F or -188.2°C), it is a liquid about 1.5 times as dense as water.
Chemical Properties
Fluorine (F) is a chemical element(group VIla, halogens).It is a pale yellow,highly toxic, corrosive, flammable gas. It is a stable, extremely strong oxidant, which may react violently with combustible materials, including plastics, reducing agents, and organic material. It reacts with water to form corrosive acids. Fluorine is very toxic and may be fatal if inhaled. Fluorine reacts violently with many oxidising agents (e.g. perchlorates, peroxides, permanganates, chlorates, nitrates, chlorine, bromine, and fluorine), strong acids (hydrochloric, sulphuric, and nitric), organic compounds, combustible materials like oil and paper, hydrogen, bromine, iodine, and chemically active metals like, potassium, sodium, magnesium, and zinc.
Fluorine is the most electro negative of all elements and the most chemically energetic of all nonmetallic elements. Fluorine is a high-tonnage chemical that is used in production of fluorides, in synthesis of fluorocarbons,and as an oxidizer for rocket fuels. Because of its severe oxidizing characteristics, special permits are required for shipping of fluorine,and all containers,piping,and processing equipment used for fluorine service must be passivated prior to use. Thereafter, they must be designated for exclusive fluorine service.
Physical properties
Fluorine does not occur in a free state in nature, and because fluorine is one of the mostreactive elements, no chemical can free it from any of its many compounds. The reason forthis is that fluorine atoms are the smallest of the halogens, meaning the electron donated by ametal (or some nonmetals) are closer to fluorine’s nucleus and thus exert a great force betweenthe fluorine nuclei and the elements giving up one electron. The positive nuclei of fluorinehave a strong tendency to gain electrons to complete the outer shell, which makes it a strongoxidizer.
Because the fluorine atom has only nine electrons, which are close to the nucleus, thepositive nucleus has a strong tendency to gain electrons to complete its outer shell. As a gasits density (specific gravity) is 1.695, and as a liquid, its density is 1.108. Its freezing point is–219.61°C, and its boiling point is –188°C. Fluorine, as a diatomic gas molecule (F2), is paleyellow in color. Fluorine is the most electronegative nonmetallic element known (wants togain electrons) and is, therefore, the strongest oxidizing agent known.
Isotopes
There are a total of 16 isotopes of fluorine. Only one, F-19, is stable. It makesup 100% of the fluorine found on Earth. All the others are radioactive with half-livesranging from 2.5 milliseconds to 4.57100×10-22 years.
Origin of Name
From the Latin and French words for “flow,” fluere.
Occurrence
Fluorine is the 13th most abundant element on the Earth. It makes up about 0.06% of theEarth’s crust. Fluorine is widely distributed in many types of rocks and minerals, but neverfound in its pure form. Fluorine is as plentiful as nitrogen, chlorine, and copper, but lessplentiful than aluminum or iron.
The most abundant fluorine mineral is fluorite—calcium fluoride (CaF2)—which is oftenfound with other minerals, such as quartz, barite, calcite, sphalerite, and galena. It is mined in Cumberland, England, and in Illinois in the United States. Other minerals from which fluorineis recovered are fluorapatite, cryolite, and fluorspar, which are found in many countriesbut mainly in Mexico and Africa.
Today fluorine is produced by the electrolysis of potassium fluoride (KF), hydrofluoric acid(HF), and molten potassium acid fluoride (KHF2).
Characteristics
Fluorine reacts violently with hydrogen compounds, including water and ammonia. It alsoreacts with metals, such as aluminum, zinc, and magnesium, sometimes bursting into flames,and with all organic compounds, in some cases resulting in such complex fluoride compoundsas fluorocarbon molecules. It is an extremely active, gaseous element that combines spontaneouslyand explosively with hydrogen, producing hydrogen fluoride acid (HF), which is usedto etch glass. It reacts with most metals except helium, neon, and argon. It forms many differenttypes of “salts” when combining with a variety of metals. Fluorine, as a diatomic gas,is extremely poisonous and irritating to the skin and lungs, as are many fluoride compounds.Fluorine and its compounds are also corrosive.
Uses
Fluorine is used in the manufacture of vari ous fluorocarbons and fluorides, as a rocketpropellant, and in many inorganic and or ganic syntheses.
Definition
Nonmetallic halogen element in group 17 of the periodic table. An 9, aw 18.99840, valence of 1, no other stable isotopes, the most electronegative element and most powerful oxidizing agent known.
General Description
Fluorine is a pale yellow gas with a pungent odor. Fluorine is commonly shipped as a cryogenic liquid. Fluorine is toxic by inhalation and skin absorption. Contact with skin in lower than lethal concentrations causes chemical burns. Fluorine reacts with water to form hydrofluoric acid and oxygen. Fluorine is corrosive to most common materials. Fluorine reacts with most combustible materials to the point that ignition occurs. Under prolonged exposure to fire or intense heat the containers may violently rupture and rocket.
Air & Water Reactions
Water vapor will react combustibly with Fluorine; an explosive reaction occurs between liquid Fluorine and ice, after an intermediate induction period, [NASA SP-3037: 52(1967)]: If liquid air, which has stood for some time is treated with Fluorine, a precipitate is formed which is likely to explode. Explosive material is thought to be Fluorine Hydrate, [Mellor 2:11(1946-1947)].
Hazard
Many of the fluorine compounds, such as CFCs, are inert and nontoxic to humans. Butmany other types of compounds, particularly the salts and acids of fluorine, are very toxicwhen either inhaled or ingested. They are also strong irritants to the skin.
There is also danger of fire and explosion when fluorine combines with several elementsand organic compounds.
Poisonous fluoride salts are not toxic to the human body at the very low concentrationlevels used in drinking water and toothpaste to prevent dental decay.
Health Hazard
reactions; highly irritating and corrosive to the eyes, skin, and mucous membranes. Toxicity The acute toxicity of fluorine is high. Even very low concentrations irritate the respiratory tract, and brief exposure to 50 ppm can be intolerable. High concentrations can cause severe damage to the respiratory system and can result in the delayed onset of pulmonary edema, which may be fatal. Fluorine is highly irritating to the eyes, and high concentrations cause severe injury and can lead to permanent damage and blindness. Fluorine is extremely corrosive to the skin, causing damage similar to second-degree thermal bums. Fluorine is not considered to have adequate warning properties. Chronic toxicity is unlikely to occur due to the corrosive effects of fluorine exposure. Fluorine has not been found to be carcinogenic or to show reproductive or developmental toxicity in humans.
Fire Hazard
Fluorine is not flammable, but is a very strong oxidizer, reacting vigorously with most oxidizable materials at room temperature, frequently with ignition. Water should not be used to fight fires involving fluorine
Flammability and Explosibility
Fluorine is not flammable, but is a very strong oxidizer, reacting vigorously with most oxidizable materials at room temperature, frequently with ignition. Water should not be used to fight fires involving fluorine.
Agricultural Uses
Fluorine is the lightest of the halogens, occurring naturally in fluorapatite, fluorite and cryolite. A pale yellow toxic gas, fluorine is made by electrolysis of potassium fluoride in liquid hydrogen fluoride. It is the most reactive, electronegative and oxidizing of all elements, and reacts with almost all elements, giving fluorides. It is used in rocket propulsion and in the production of uranium and fluorocarbons.
Materials Uses
Nickel, iron, aluminum, magnesium, copper, and certain of their alloys are quite satisfactory for handling fluorine at room temperature, for these are among the metals with which formation of a surface fluoride film retards further reaction.
Potential Exposure
Elemental fluorine is used in the con version of uranium tetrafluoride to uranium hexafluoride; in the synthesis of organic and inorganic fluorine com pounds; and as an oxidizer in rocket fuel.
Environmental Fate
Fluorine remains persistent in the environment. In water, fluorides attach to aluminum in freshwater and calcium and magnesium in seawater and settle into the sediment. Fluorides may be taken up from soil and accumulate in plants or they may be deposited on the upper parts of the plants. The amount of fluoride taken up by plants depends on the type of plant, the nature of the soil, and the amount and form of fluoride in the soil. Levels of fluorides in surface water average about 0.2 ppm, while well water levels range from 0.02 to 1.5 ppm. The 15 000 water systems serving about 162 million people in the USA are fluoridated in the range of 0.7–1.2 ppm.
storage
Work with fluorine requires special precautions and protective equipment and should be carried out only by specially trained personnel. Fluorine will react with many materials normally recommended for handling compressed gases.
Shipping
UN1045 Fluorine, compressed, Hazard Class: 2.3; Labels: 2.3-Poisonous gas, 5.1-Oxidizer, 8-Corrosive material, Inhalation Hazard Zone A. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written per mission of the owner.
Purification Methods
Pass the gas through a bed of NaF at 100o to remove HF and SiF4. [For description of stills used in fractional distillation, see Greenberg et al. J Phys Chem 65 1168 1961; Stein et al. Purification of Fluorine by Distillation, Argonne National Laboratory, ANL-6364 1961 (from Office of Technical Services, US Dept of Commerce, Washington 25).] HIGHLY TOXIC.
Incompatibilities
Fluorine is an extremely powerful oxi dizing gas. Keep away from heat, water, nitric acid, oxidi zers, organic compounds. Containers may explode if heated. Reacts violently with reducing agents; ammonia, all combustible materials, metals (except the metal containers in which it is shipped). Reacts violently with H2O to form hydrofluoric acid, oxygen and ozone. The most potent oxidizer.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Fluorine may be combusted by means of a fluorine-hydrocarbon air burner followed by a caustic scrubber and stack. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations gov erning storage, transportation, treatment, and waste disposal.
Fluorine Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
chevron_right7782-41-4(Fluorine)Related Search:
1of4
chevron_right




