Ascoric Acid
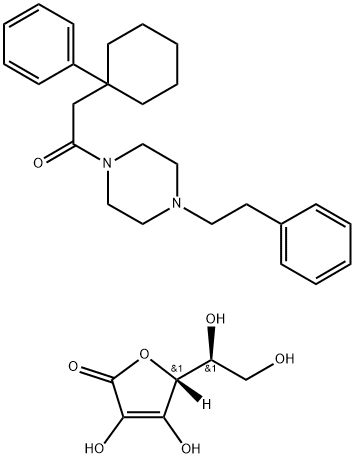
- CAS No.
- 36431-82-0
- Chemical Name:
- Ascoric Acid
- Synonyms
- Ascoric Acid;Ascoric Acid USP/EP/BP;1-(4-phenethylpiperazin-1-yl)-2-(1-phenylcyclohexyl)ethanoneascorbate;Piperazine, 1-((1-phenylcyclohexyl)acetyl)-4-(2-phenylethyl)-, coMpd. with L-ascorbic acid
- CBNumber:
- CB32130241
- Molecular Formula:
- C32H42N2O7
- Molecular Weight:
- 566.7
- MOL File:
- 36431-82-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/4 15:16:29
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS08,GHS07,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H411-H336-H304-H319 |
| Precautionary statements | P261-P271-P304+P340-P312-P403+P233-P405-P501-P264-P280-P305+P351+P338-P337+P313P |
Ascoric Acid Chemical Properties,Uses,Production
Description
Ascorbic acid is a naturally occurring organic compound with antioxidant properties. It is a white solid, but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions. Ascorbic acid is one form ("vitamer") of vitamin C. It was originally called L-hexuronic acid, but when it was found to have vitamin C activity in animals ("vitamin C" being defined as a vitamin activity, not then a specific substance), the suggestion was made to rename L-hexuronic acid. The new name for L-hexuronic acid is derived from a- (meaning "no") and scorbutus (scurvy), the disease caused by a deficiency of vitamin C. Because it is derived from glucose, many animals are able to produce it, but humans require it as part of their nutrition. Other vertebrates lacking the ability to produce ascorbic acid include other primates, guinea pigs, teleost fishes, bats,and birds, all of which require it as a dietary micronutrient (that is, a vitamin).
Chemical Properties
Ascorbic acid is present in nutritionally useful amounts in many edible plants, especially in rapidly growing leafy vegetables, fruits, tomatoes and potatoes. Foods of animal origin as usually consumed are generally poor sources of the vitamin. Of the stereoisomers (L-ascorbic acid, D-ascorbic acid, L-isoascorbic acid and D-isoascorbic acid or erythorbic acid), only L-ascorbic acid has significant vitamin C activity. The vitamin C activity of ascorbyl palmitate is approximately equal to that of L-ascorbic acid. Ascorbic acid has a pleasant, sharp, acidic taste without any odor. It is extensively used as antioxidant, meat-curing aid, nutrient and dietary supplement. For a detailed description, see Burdock (1997).
Occurrence
Reported found in rose hip, black currants, the juice of citrus fruits and the ripe fruit of Capsicum annuum L.
History
From the middle of the 18th century, it was noted that lemon juice could prevent sailors from getting scurvy. At first, it was supposed that the acid properties were responsible for this benefit; however, it soon became clear that other dietary acids, such as vinegar, had no such benefits. In 1907, two Norwegian physicians reported an essential disease-preventing compound in foods that was distinct from the one that prevented beriberi. These physicians were investigating dietary deficiency diseases using the new animal model of guinea pigs, which are susceptible to scurvy. The newly discovered food-factor was eventually called vitamin C.
From 1928 to 1932, the Hungarian research team led by Albert Szent-Gy?rgyi, as well as that of the American researcher Charles Glen King, identified the antiscorbutic factor as a particular single chemical substance. At the Mayo clinic, Szent-Gy?rgyi had isolated the chemical hexuronic acid from animal adrenal glands. He suspected it to be the antiscorbutic factor, but could not prove it without a biological assay. This assay was finally conducted at the University of Pittsburgh in the laboratory of King, which had been working on the problem for years, using guinea pigs. In late 1931, King's lab obtained adrenal hexuronic acid indirectly from Szent-Gy?rgyi and using their animal model, proved that it is vitamin C, by early 1932.
Uses
Vitamin (antiscorbutic); acidifier (urinary).
Preparation
Ascorbic acid is prepared industrially from glucose in a method based on the historical Reichstein process. In the first of a five-step process, glucose is catalytically hydrogenated to sorbitol, which is then oxidized by the microorganism Acetobacter suboxydans to sorbose. Only one of the six hydroxy groups is oxidized by this enzymatic reaction. From this point, two routes are available. Treatment of the product with acetone in the presence of an acid catalyst converts four of the remaining hydroxyl groups to acetals. The unprotected hydroxyl group is oxidized to the carboxylic acid by reaction with the catalytic oxidant TEMPO (regenerated by sodium hypochlorite — bleaching solution). (Historically, industrial preparation via the Reichstein process used potassium permanganate.) Acid-catalyzed hydrolysis of this product performs the dual function of removing the two acetal groups and ring-closing lactonization. This step yields ascorbic acid. Each of the five steps has a yield larger than 90 %.
Reactions
Ascorbic acid resembles the sugar from which it is derived, being a ring with many oxygen-containing functional groups. The molecule exists in equilibrium with two ketone tautomers, which are less stable than the enol form . In solutions, these forms of ascorbic acid rapidly interconvert.
Nucleophilic attack of ascorbic enol on proton to give 1,3-diketone.
Biosynthesis
Ascorbic acid is found in plants and animals where it is produced from glucose. Animals must either produce it or digest it, otherwise a lack of vitamin C may cause scurvy which may eventually lead to death. Reptiles and older orders of birds make ascorbic acid in their kidneys. Recent orders of birds and most mammals make ascorbic acid in their liver where the enzyme L-gulono lactone oxidase is required to convert glucose to ascorbic acid. Humans, some other primates, and guinea pigs are not able to make L-gulono lactone oxidase because of a genetic mutation and are therefore unable to make ascorbic acid. Synthesis and signalling properties are still under investigation.
Mechanism of action
As a mild reducing agent, ascorbic acid degrades upon exposure to air, converting the oxygen to water. The redox reaction is accelerated by the presence of metal ions and light. It can be oxidized by one electron to a radical state or doubly oxidized to the stable form called dehydroascorbic acid.
Ascorbate usually acts as an antioxidant. It typically reacts with oxidants of the reactive oxygen species, such as the hydroxyl radical formed from hydrogen peroxide. Such radicals are damaging to animals and plants at the molecular level due to their possible interaction with nucleic acids, proteins, and lipids. Sometimes these radicals initiate chain reactions. Ascorbate can terminate these chain radical reactions by electron transfer. Ascorbic acid is special because it can transfer a single electron, owing to the stability of its own radical ion called "semidehydroascorbate", dehydroascorbate. The net reaction is :
RO? + C6H7O6- → ROH + C6H6O6?-
The oxidized forms of ascorbate are relatively unreactive, and do not cause cellular damage.
However, being a good electron donor, excess ascorbate in the presence of free metal ions can not only promote but also initiate free radical reactions, thus making it a potentially dangerous pro-oxidative compound in certain metabolic contexts.
Acidity
Ascorbic acid, a reductone, behaves as a vinylogous carboxylic acid wherein the electrons in the double bond, hydroxyl group lone pair, and the carbonyl double bond form a conjugated system. Because the two major resonance structures stabilize the deprotonated conjugate base of ascorbic acid, the hydroxyl group in ascorbic acid is much more acidic than typical hydroxyl groups. In other words, ascorbic acid can be considered an enol in which the deprotonated form is a stabilized enolate.
Ascoric Acid Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| AMOLI ORGANICS PVT LTD | +91-22-6621 4715 | New Delhi, India | 21 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21676 | 55 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 26267 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 49999 | 58 | Inquiry |
| Hubei Enxing Biotechnology Co., Ltd | 16621771607 | China | 8329 | 58 | Inquiry |
| Beijing Solarbio Science & Tecnology Co., Ltd. | 010-50973186 4009686088 | China | 18352 | 68 | Inquiry |
| CSPC WEISHENG PHARMACEUTICAL SHIJIAZHUANG CO LTD | 0311-85966833 | China | 2 | 58 | Inquiry |
| DSM NUTRITIONAL PRODUCTS LTD | +41 79 260 85 22 | Switzerland | 15 | 58 | Inquiry |
| DSM Nutritional Products Ltd. | 01773 536500 | Switzerland | 23 | 58 | Inquiry |
| CSPC WEISHENG PHARMACEUTICAL (SHIJIAZHUANG) CO., LTD | 0311-85966833 | China | 2 | 58 | Inquiry |





