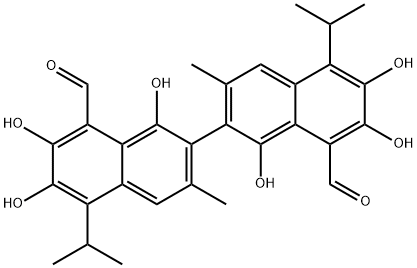
GOSSYPOL
- CAS No.
- 303-45-7
- Chemical Name:
- GOSSYPOL
- Synonyms
- GOSSYPOL(P);ssypol;BL 193;Pogosin;GOSSYPOL;Thespesin;NSC 56817;NSC 624336;Gossypol solution;GOSSYPOL USP/EP/BP
- CBNumber:
- CB2770457
- Molecular Formula:
- C30H30O8
- Molecular Weight:
- 518.55
- MOL File:
- 303-45-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:04
| Melting point | 181-183 C |
|---|---|
| Boiling point | 522.63°C (rough estimate) |
| Density | 1.1912 (rough estimate) |
| refractive index | 1.4900 (estimate) |
| storage temp. | 2-8°C |
| solubility | ≥25.95 mg/mL in DMSO; insoluble in H2O; ≥2.1 mg/mL in EtOH |
| form | Off-white to yellow solid. |
| pka | 7.15±0.50(Predicted) |
| color | Light yellow to yellow |
| Water Solubility | Soluble in 100%ethanol (25 mg/ml), DMF (25 mg/ml), acetone, DMSO (25 mM), methanol (2 mg/ml), ether, chloroform, sodium carbonate, and dilute aqueous solutions of ammonia . Insoluble in water. Gossypol is a male antifertility agent with antispermatogenic activity and has been shown to contain antitumor, anitviral, and antioxidant properties. |
| InChIKey | QBKSWRVVCFFDOT-UHFFFAOYSA-N |
| LogP | 5.419 (est) |
| CAS DataBase Reference | 303-45-7 |
| EPA Substance Registry System | [2,2'-Binaphthalene]-8,8'-dicarboxaldehyde, 1,1',6,6',7,7'-hexahydroxy-3,3'-dimethyl-5,5'-bis(1-methylethyl)- (303-45-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H360F | |||||||||
| Precautionary statements | P201-P202-P264-P270-P301+P312-P308+P313 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-40-36/37/38 | |||||||||
| Safety Statements | 22-36-36/37/39-27-26 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | DU3100000 | |||||||||
| HS Code | 29124990 | |||||||||
| Toxicity | A secondary plant product produced by some varieties of cotton and found in cottonseed meal and cottonseed oil from those varieties. Affects the male reproductive system and has potential use as a male contraceptive agent. May be irritating to the GI tract. In animal studies large doses caused lung edema and paralysis. | |||||||||
| NFPA 704 |
|
GOSSYPOL price More Price(2)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | G8761 | (±)-Gossypol from cotton seeds ≥95% (HPLC) | 303-45-7 | 100MG | ₹20751.53 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | G8761 | (±)-Gossypol from cotton seeds ≥95% (HPLC) | 303-45-7 | 250MG | ₹41568 | 2022-06-14 | Buy |
GOSSYPOL Chemical Properties,Uses,Production
Description
Gossypol is mainly present in the roots, stems, leaves, and seeds of the plant cotton, and the highest content is found in cotton seeds. Its chemical structure was first determined by Adams in 1938 and was used to be studied in antitumor research.
Chemical Properties
Soild
Physical properties
Appearance: plate or needle-like crystals with yellow color, odorless, and tasteless. Solubility: do not dissolve in water, slightly soluble in ethanol, and soluble in chloroform, ether, acetone, ethyl acetate, dichloroethane, carbon tetrachloride, and pyridine. Difficult to dissolve in cyclohexane, benzene, and petroleum ether. Melting point: 184–214?°C.
Gossypol has a chiral structure with two optical isomers in the left and right. The formic acid gossypol or gossypol acetate was commonly used.
Occurrence
Gossypol is found in cotton and is made synthetically.
History
The close relationship between male infertility and the consumption of cottonseed products was first found in cotton-producing areas by Shandong scientists in 1971. Further study found that gossypol impaired the sperm quality significantly in rat, rabbit, and so on. Mechanism study showed that gossypol could interact with sperm and then trigger a cascade reaction for different stages of spermatogenesis. Acetate gossypol showed similar biological activities with gossypol. The clinical trial for gossypol tablet for the male contraceptive treatment was initiated in the 1973, which was produced in Shanghai, and the raw material was provided from Shanghai, Shandong, and Zhejiang province. During 1981–1985, the side effect, antifertility reversibility, and safety evaluation induced by gossypol were further investigated by the national 65-35-2-5 scientific group, which was carried out by prospective clinical study in 390 volunteers, and the results showed that there is no carcinogenic, teratogenic, and mutagenic effects in gossypol .
Uses
Gossypol is a mixture of natural polyphenols isolated from the cotton plant. It causes spermatogenesis arrest in humans and also functions as an anti-malarial agent. Antiinflammatory. Anticancer.
Indications
This product as gossypol acetate is recorded in the tenth volume of national standards for chemical drugs.
Solid formulations include compound acetate gossypol tablets and gossypol potassium chloride vitamin B capsule. Gossypol was used as male contraceptive drug in clinic previously but terminated due to the induced severer hypokalemia. Now, compound acetate gossypol tablets consisted of acetate gossypol and potassium chloride, vitamins are used for the treatment of uterine functional bleeding, uterine fibroids, and menorrhagia and endometriosis.
General Description
Gossypol is considered as an antifertility agent. It is present in cottonseed as a yellowish pigment. This reactive sesquiterpene aldehyde is present in the family Malvaceae and has a molecular mass of 518.54.
Hazard
Toxic by ingestion but is inactivated by heat, 0.04% max allowed in foods.
Biological Activity
Lipophilic agent derived from cottonseed. Exhibits multiple biological effects including antifertility and anticancer activity. Pro-apoptotic; downregulates Bcl-2 and Bcl-XL.
Pharmacology
There are significant differences in pharmacological effects of gossypol between
male and female. In males, gossypol could significantly reduce sperm quality, as
manifested by the reduced sperm density or activity. Mechanism study showed that
gossypol could impair spermatogenic epithelium directly, and sperm cells and spermatocytes are the most sensitive. In female, gossypol could inhibit ovarian and
endometrial, muscular steroid hormone receptor, hence thinning the endometrium
and muscle layer and reducing menstrual flow ; therefore, gossypol acetate and
potassium chloride, vitamins, and other combinations were prepared for the compound acetate gossypol tablets, which were used for the treatment of uterine functional bleeding, uterine fibroids, menorrhagia, and uterus endometriosis. The
recommended daily oral dose is 20 mg. Gossypol dextrorate has no effect, with
levorotate as the active ingredient; therefore, the role of L-gossypol is two times that
of gossypol .
A good antitumor effect of gossypol and its derivatives was found in recent studies. Gossypol showed significant inhibition on cancer cell proliferation in vitro studies, including tumor cells derived from lymphatic and granulocytes, adrenal glands,
breast, and cervical, rectal, and central nervous system. Studies have suggested that
gossypol inhibits the catalytic activity of topoisomerase II and intervenes in the
stability of topoisomerase – DNA complexes. Gossypol blocks the cell in S stage by
reducing the activity of DNA polymerase alpha and beta and inhibiting DNA synthesis. Gossypol is a specific DNA synthesis inhibitor that inhibits cell division. In
addition, gossypol can also exhibit antitumor effect by acting as a signal pathway
regulator and inhibiting the energy metabolism of tumor cells .
Clinical Use
Due to the presence of hypokalemia and irreversible antifertility risk of gossypol, the clinical indications of male contraceptives are not approved by the Chinese Food and Drug Administration. At present, gossypol and its derivatives are mainly used for female uterine fibroids, functional uterine bleeding, and endometriosis treatment. In addition, the treatment of gossypol and its derivatives in the tumor is gradually confirmed clinically, such as gossypol combined with docetaxel or cisplatin for the treatment of malignant non-small cell lung cancer, and is currently in phase III clinical trial . Gossypol combined with rituximab can achieve better therapeutic efficacy against granulocytic leukemia and is currently in phase II clinical trials .
GOSSYPOL Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21669 | 55 | Inquiry |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 | CHINA | 1824 | 58 | Inquiry |
| Chengdu Biopurify Phytochemicals Ltd. | +86-28-82633860 +86-18080483897 | China | 3424 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | China | 11616 | 58 | Inquiry |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 | China | 13127 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | China | 34571 | 58 | Inquiry |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | China | 23556 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 9030 | 58 | Inquiry |
303-45-7(GOSSYPOL)Related Search:
1of4
chevron_right




