Magnesium diamide
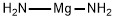
- CAS No.
- 7803-54-5
- Chemical Name:
- Magnesium diamide
- Synonyms
- magnesium amide;Diaminomagnesium;Magnesium diamide
- CBNumber:
- CB2852227
- Molecular Formula:
- H4MgN2
- Molecular Weight:
- 56.35
- MOL File:
- 7803-54-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/17 17:11:56
| Melting point | decomposes when heated [HAW93] |
|---|---|
| Density | d425 1.39 |
| solubility | reacts with H2O |
| form | white powder |
| color | white powder; flammable |
| Water Solubility | reacts violently with H2O releasing NH3 [MER06] |
| EPA Substance Registry System | Magnesium amide (Mg(NH2)2) (7803-54-5) |
Magnesium diamide Chemical Properties,Uses,Production
Chemical Properties
Whitish to gray crystals. D 1.40. Decomposes when heated.
Uses
Polymerization catalyst.
General Description
A whitish to gray-colored crystalline solid. Denser than water. Contact may irritate skin, eyes and mucous membranes. Vapors may be toxic by inhalation. Used to make other chemicals.
Air & Water Reactions
May spontaneously ignite upon exposure to air. Soluble in water. Reacts violently with water to form caustic ammonia/ammonium hydroxide and heat.
Reactivity Profile
Bases or alkalis are chemically similar to sodium hydroxide (NaOH) or sodium oxide (Na2O). They neutralize acids exothermically to form salts plus water. When soluble in water they give solutions having a pH greater than 7.0. Mixing these materials with water can generate troublesome amounts of heat as the base is dissolved or diluted. Bases react with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. Bases may initiate polymerization reactions in polymerizable organic compounds, especially epoxides). They may generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. Materials of this group often serve as catalysts.
Hazard
A pyrophoric material igniting in air at room temperature. Evolves ammonia on vigorous reaction with water.
Health Hazard
Fire will produce irritating, corrosive and/or toxic gases. Inhalation of decomposition products may cause severe injury or death. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.
Fire Hazard
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.
Magnesium diamide Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21675 | 55 | Inquiry |
| Jiangsu Raien Environmental Protection Technology Co., Ltd | 0513-66814573 16651336298 | China | 2995 | 58 | Inquiry |
| HONEST JOY HOLDINGS LIMITED | +86-755-26404303 | United States | 6702 | 54 | Inquiry |
| Hubei Lidu New Material Technology Co., Ltd. | 0724-4355788 19307246857 | China | 752 | 58 | Inquiry |
| Shaanxi Didu New Material Co., Ltd | 029-63373950 15353716720 | China | 10011 | 58 | Inquiry |
7803-54-5(Magnesium diamide)Related Search:
1of4
chevron_right




