MAGNESIUM SILICIDE
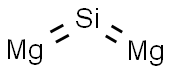
- CAS No.
- 22831-39-6
- Chemical Name:
- MAGNESIUM SILICIDE
- Synonyms
- PubChem ID: 89858;MAGNESIUM SILICIDE;dimagnesium silicide;magnesiumsilicide(mg2si);Magnesium silicide MgSi2;MAGNESIUM SILICIDE -45 MY;Magnesium silicide, 99.5%;Silanediylidenedimagnesium;Magnesium silicide, powder;MagnesiuM silicide, 99.99%
- CBNumber:
- CB3115750
- Molecular Formula:
- Mg2Si
- Molecular Weight:
- 76.7
- MOL File:
- 22831-39-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/14 15:18:26
| Melting point | 1102 °C |
|---|---|
| Density | 1,94 g/cm3 |
| solubility | reacts with H2O |
| form | Powder |
| Specific Gravity | 2. |
| color | Blue |
| Water Solubility | Insoluble in water and denser than water. |
| Sensitive | Air & Moisture Sensitive |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| Crystal Structure | Cubic, Antifluorite Structure - Space Group Fm3m |
| Merck | 14,5688 |
| Stability | Spontaneously flammable - mixtures with air are explosive. Reacts with water to form toxic vapours. |
| EPA Substance Registry System | Magnesium silicide (Mg2Si) (22831-39-6) |
MAGNESIUM SILICIDE price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 752630 | Magnesium silicide pieces, 99.7% trace metals basis | 22831-39-6 | 5G | ₹3344.93 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 343196 | Magnesium silicide ≥99% trace metals basis, ?20?mesh | 22831-39-6 | 25G | ₹6679.03 | 2022-06-14 | Buy |
| ALFA India | ALF-012837-18 | Magnesium silicide, 99.5% (metals basis) | 22831-39-6 | 50g | ₹20691 | 2022-05-26 | Buy |
| ALFA India | ALF-012837-09 | Magnesium silicide, 99.5% (metals basis) | 22831-39-6 | 10g | ₹4887 | 2022-05-26 | Buy |
MAGNESIUM SILICIDE Chemical Properties,Uses,Production
Description
Magnesium silicide has the molecular formula of
Mg2Si and the molecular weight of 76.6955 g/mol. Its density is 1.998 g/cc
and its melting point is 1102°C. It is a black powder.
Mg2Si crystallizes in a cubic CaF2 (fluorspar)-type
lattice with a = 4.49 ? . The centers of this lattice are analogous
to that of diamond. Each Si atom forms four covalent
sp3 bonds. The Mg subshells are only half-filled.
There is no bonding between the two Mg atom sites.
The distance between the Mg and Si atoms is 2.77 ? .
Bonding in this compound is essentially covalent.
Chemical Properties
Magnesium silicide is a slate blue crystalline solid.
Physical properties
Magnesium silicide is unstable in air and reacts with moisture to form silane. When magnesium silicide is placed into aqueous HCl, the gas silane, SiH4, is produced. This gas is the silicon analogue of methane, CH4, but is more reactive. Silane is pyrophoric, that is due to the presence of O2 in the air, i.e. it spontaneously combusts in air. These reactions are typical of a Group-2 silicide. Mg2Si reacts similarly with sulfuric acid. As a powder, magnesium silicide is dark blue or slightly purple in color.
Uses
Magnesium silicide is used to produce alloys such as aluminum alloys. It is also used in food, beverages, as a flavor enhancer and as an intermediate in chemical research.
General Description
A colorless crystalline solid. Insoluble in water and denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion.
Air & Water Reactions
Contact with moisture under acidic condition generates silanes that ignite in air. Insoluble in water.
Reactivity Profile
MAGNESIUM SILICIDE is a reducing agent. May react vigorously with oxidizing materials.
Health Hazard
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard.
Potential Exposure
Magnesium silicide is used in the semiconductor industry and to produce certain aluminum alloys
Shipping
UN2624 Magnesium silicide, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet materia
Structure and conformation
The space lattice of Mg2Si belongs to the cubic system, and it has an antifluorite type structure (refer to Mg2Ge) with a lattice constant of a=0.634 nm.
Incompatibilities
Possibly pyrophoric, especially in moist air. Pyrophoric; mixtures with air are spontaneously explosive. A strong reducing agent. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, mineral acids, strong acids, strong bases. Reacts with water; releasing explosive hydrogen gas and may also release selfigniting toxic silane gas
MAGNESIUM SILICIDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6700 | 30 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6905 | 58 | Inquiry |
| Aritech Chemazone Private Limited | +91-9034345475 | Punjab, India | 684 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29884 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39894 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 7724 | 58 | Inquiry |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | China | 52849 | 58 | Inquiry |
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 | China | 2904 | 58 | Inquiry |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 | China | 18154 | 58 | Inquiry |
| PT CHEM GROUP LIMITED | China | 35425 | 58 | Inquiry |
22831-39-6(MAGNESIUM SILICIDE)Related Search:
1of4
chevron_right





