Radotinib
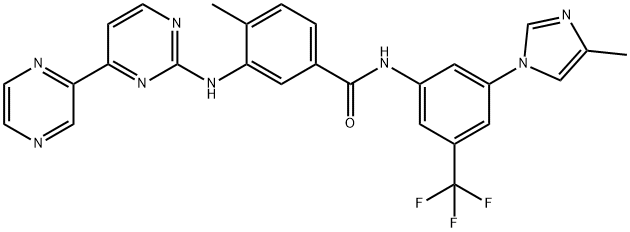
- CAS No.
- 926037-48-1
- Chemical Name:
- Radotinib
- Synonyms
- Supect;Radotinib HCl;IY5511;CS-1712;Radotinib;IY5511 HCl.;Radotinib(IY-5511);RADOTINIB; IY5511 HCL.;Testosterone Impurity 43;4-methyl-N-[3-(4-methylimidazol-1-yl)-5-(trifluoromethyl)phe...
- CBNumber:
- CB32667981
- Molecular Formula:
- C27H21F3N8O
- Molecular Weight:
- 530.5
- MOL File:
- 926037-48-1.mol
- Modify Date:
- 2024/7/2 8:55:06
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H315-H318-H334-H317-H341-H361-H370-H335-H413 | |||||||||
| Precautionary statements | P201-P202-P260-P264-P270-P272-P273-P280-P284-P301+P312-P302+P352-P304+P341-P305+P351+P338-P310-P308+P313-P321-P330-P362+P364-P333+P313-P342+P311-P363-P405-P501 | |||||||||
| NFPA 704 |
|
Radotinib Chemical Properties,Uses,Production
Description
Radotinib, an inhibitor of Bcr–Abl tyrosine kinase,was approved in January 2012 in Korea as a second-line treatment for chronic myeloid leukemia (CML). Radotinib is a TKI with a similar structure to the second-generation TKI, nilotinib, in which a pyridyl group has been replaced with a pyrazinemoiety. The in vitro activity of radotinib against a variety of tumor cell lines is disclosed in an issued patent. Radotinib was significantly more potent than imatinib in all of the cell lines tested. The synthesis of radotinib via amide coupling is described in the patent literature.
Uses
Radotinib is tyrosine kinase inhibitor. In a biological study, it can induce cytotoxicity in c-KIT-positive malignancies including acute myeloid leukemia and small cell lung cancer in human making it potential target agent for treatment of such malignancies. It is a COVID19-related research product.
Indications
Radotinib (Supect(R), Il-Yang Pharmaceutical) is a Bcr–Abl inhibitor that was approved in South Korea in 2012 for the treatment of imatinib-resistant CML. Radotinib, which has a terminal 4-(pyridine-2-yl) pyrimidine moiety, was developed based on the previously approved Bcr–Abl inhibitors nilotinib. Radotinib has equivalent efficacy with that of other second-generation Bcr–Abl inhibitors and is well tolerated in chronic-phase CML patients. The lower cost of radotinib compared with other FDA-approved Bcr–Abl inhibitors makes it an attractive alternative for the treatment of CML in developing nations.
Radotinib Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32760 | 60 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | China | 10326 | 58 | Inquiry |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | China | 10522 | 58 | Inquiry |
| InvivoChem | +1-708-310-1919 +1-13798911105 | United States | 6393 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418684 +8618949823763 | China | 25363 | 58 | Inquiry |
926037-48-1(Radotinib)Related Search:
1of2
chevron_right




