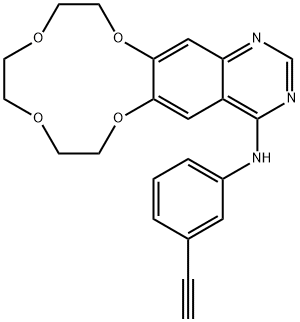
Icotinib
- CAS No.
- 610798-31-7
- Chemical Name:
- Icotinib
- Synonyms
- ConMana;N-(3-Ethynylphenyl)-7,8,10,11,13,14-hexahydro-[1,4,7,10]tetraoxacyclododecino[2,3-g]quinazolin-4-amine;otinib;CS-908;BP-1096;BPI-2009;Icotinib;Lcotinib;BPI-2009H;Icotinib BP-1096
- CBNumber:
- CB82616025
- Molecular Formula:
- C22H21N3O4
- Molecular Weight:
- 391.42
- MOL File:
- 610798-31-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/4/17 18:22:24
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P330-P501 | |||||||||
| HS Code | 29339900 | |||||||||
| NFPA 704 |
|
Icotinib Chemical Properties,Uses,Production
Description
Icotinib is also known as ConMana. It is a highly selective, first-generation inhibitor of epidermal growth factor receptor tyrosine kinase (EGFR-TKI), whose mutation and overexpression is involved in many kinds of cancer. Icotinib is a quinazoline derivative that binds reversibly to the ATP binding site of the EGFR protein, being capable of blocking the signal transduction cascade. It is currently under investigation for its treatment efficacy on the EGFR+ Non-small cell lung cancer.
Uses
Icotinib is a potent and specific epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) with IC50 of 5 nM.
Indications
Icotinib (Conmana?, BetaPharma), an EGFR inhibitor, was approved by the China State FDA in 2011 for the treatment of NSCLC. Icotinib resembles erlotinib in possessing the N-(3-ethylnylphenyl) quinazolin-4-amine core scaffold of erlotinib that binds to the EGFR ATP pocket. An adjacent hydrophobic group was also retained while the solvent exposed two 2-methoxyethyoxysubstituents at the 6- and 7-positions of the quinazoline core were cyclized to afford the tetraoxacyclododecene moiety of icotinib. Efficacy and safety of icotinib as first-line therapy in patients has been evaluated for advanced NSCLC in recent clinical studies.
General Description
Class: receptor tyrosine kinase
Treatment: NSCLC
Oral bioavailability = 52%
Elimination half-life = 5.5 h
References
Tan, F., et al. "Icotinib (BPI-2009H), a novel EGFR tyrosine kinase inhibitor, displays potent efficacy in preclinical studies. " Lung Cancer76.2(2012):177-82.
Shi, Y., et al. "Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial." Lancet Oncology 14.10(2013):953-61.
Sun, Y., Y. Shi, and L. Zhang. "A randomized, double-blind phase III study of icotinib versus gefitinib in patients with advanced non-small cell lung cancer (NSCLC) previously treated with chemotherapy (ICOGEN)."Journal of Thoracic Oncology 29.15(2011):-.
https://en.wikipedia.org/wiki/Icotinib
Icotinib Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | China | 29730 | 60 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29859 | 58 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19552 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 32159 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 22854 | 58 | Inquiry |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | China | 9642 | 58 | Inquiry |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | China | 9304 | 58 | Inquiry |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | China | 10473 | 58 | Inquiry |
610798-31-7(Icotinib)Related Search:
1of4
chevron_right




