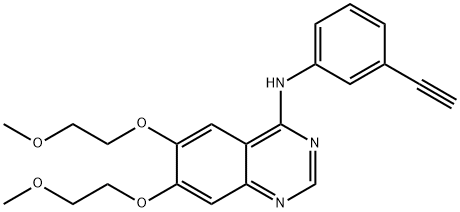
Erlotinib
- CAS No.
- 183321-74-6
- Chemical Name:
- Erlotinib
- Synonyms
- ERLOTINIB HCL;Tarceva;Erlortinib;R 1415;Rotini;OSI 744;Erlonat;ERLOTININ;ERLOTINIB;NSC-71878
- CBNumber:
- CB9285914
- Molecular Formula:
- C22H23N3O4
- Molecular Weight:
- 393.44
- MOL File:
- 183321-74-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 159-160 °C |
|---|---|
| Boiling point | 553.6±50.0 °C(Predicted) |
| Density | 1.24 |
| storage temp. | Keep in dark place,Sealed in dry,Store in freezer, under -20°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 5.32±0.30(Predicted) |
| color | White |
| CAS DataBase Reference | 183321-74-6(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H350-H304-H227 |
| Precautionary statements | P501-P202-P210-P201-P280-P370+P378-P331-P308+P313-P301+P310-P403+P235-P405 |
| Safety Statements | 24/25 |
| HS Code | 29335990 |
| Hazardous Substances Data | 183321-74-6(Hazardous Substances Data) |
Erlotinib Chemical Properties,Uses,Production
Description
Erlotinib is a tyrosine kinase inhibitor which acts on the epidermal growth factor receptor (EGFR), inhibiting EGFR-
Uses
Erlotinib HCl is an HER1/EGFR inhibitor with IC50 of 2 nM.
Definition
ChEBI: A quinazoline compound having a (3-ethynylphenyl)amino group at the 4-position and two 2-methoxyethoxy groups at the 6- and 7-positions.
General Description
Class: receptor tyrosine kinase
Treatment: NSCLC
Oral bioavailability = 60%
Elimination half-life = 36 h
Protein binding = 93%
Side effects
- Burning, tingling, numbness or pain in the hands, arms, feet, or legs.
- cough or hoarseness.
- diarrhea (severe)
- difficult or labored breathing.
- fever or chills.
- rash (severe)
- sensation of pins and needles.
- stabbing chest pain.
Synthesis
The synthesis of Erlotinib is as follows:
N-dimethylformamidine, 0.72 g (6.15 mmol) of N '- [2-cyano-4,5-bis (2-methoxyethoxy) phenyl] ) Of 3-aminophenylacetylene and 8 mL of acetic acid were reacted in a 50 mL reaction flask at 125 ° C for 1 hour and cooled to room temperature.20 mL of ice water was added to the mixture, the pH was adjusted to 10 with aqueous ammonia, and the mixture was stirred for 1 hour, suction filtered and the filter cake washed with water until neutral.The filter cake was dried to obtain 2.15 g of erlotinib in a yield of 91.5%.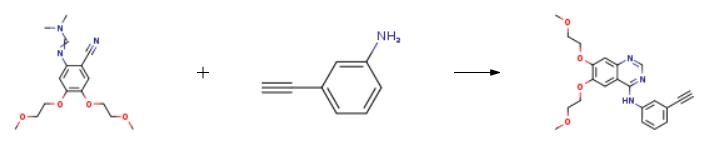
Erlotinib Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| Gonane Pharma | +91-9819380043 +91-9819380043 | NaviMumbai, India | 192 | 58 | Inquiry |
| Vivimed Labs Ltd | +914066086608 | Telangana, India | 61 | 58 | Inquiry |
| Laurus Labs Ltd | +91-4066594333 +91-4039804333 | Telangana, India | 50 | 58 | Inquiry |
| Sakar Healthcare | +91-8976292690 +91-9967572302 | Gujarat, India | 47 | 58 | Inquiry |
| Shreyaa Medilife Private Limited | +91-6354474696 +91-9879513108 | Ahmedabad, India | 67 | 58 | Inquiry |
| Aspen Biopharma Labs Pvt Ltd | +91-9248058660 +91-9248058662 | Telangana, India | 234 | 58 | Inquiry |
| AKASH PHARMA EXPORTS | +91-9388123451 +91-9846039283 | Kerela, India | 470 | 58 | Inquiry |
| Medicare Remedies Private Limited | 08048957356 | Mumbai, India | 8 | 58 | Inquiry |
| Real Chemist | 08048612528 | Mumbai, India | 2 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Jigs Chemical ltd | 58 |
| Gonane Pharma | 58 |
| Vivimed Labs Ltd | 58 |
| Laurus Labs Ltd | 58 |
| Sakar Healthcare | 58 |
| Shreyaa Medilife Private Limited | 58 |
| Aspen Biopharma Labs Pvt Ltd | 58 |
| AKASH PHARMA EXPORTS | 58 |
| Medicare Remedies Private Limited | 58 |
| Real Chemist | 58 |





