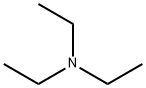
Triethylamine
- CAS No.
- 121-44-8
- Chemical Name:
- Triethylamine
- Synonyms
- TEA;Et3N;N,N-DIETHYLETHANAMINE;(C2H5)3N;TEN;Triethylamin;Trietilamina;TRIEHYLAMINE;N,N,N-Triethylamine;N,N-Diethylethanamin
- CBNumber:
- CB5355941
- Molecular Formula:
- C6H15N
- Molecular Weight:
- 101.19
- MOL File:
- 121-44-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/24 19:19:26
| Melting point | -115 °C |
|---|---|
| Boiling point | 90 °C |
| Density | 0.728 |
| vapor density | 3.5 (vs air) |
| vapor pressure | 51.75 mm Hg ( 20 °C) |
| FEMA | 4246 | TRIETHYLAMINE |
| refractive index |
n |
| Flash point | 20 °F |
| storage temp. | Store below +30°C. |
| solubility | water: soluble112g/L at 20°C |
| pka | 10.75(at 25℃) |
| form | Liquid |
| Specific Gravity | 0.725 (20/4℃) |
| color | Clear |
| PH | 12.7 (100g/l, H2O, 15℃)(IUCLID) |
| Relative polarity | 1.8 |
| Odor | Strong ammonia-like odor |
| Odor Type | fishy |
| Odor Threshold | 0.0054ppm |
| explosive limit | 1.2-9.3%(V) |
| Water Solubility | 133 g/L (20 ºC) |
| JECFA Number | 1611 |
| Merck | 14,9666 |
| BRN | 1843166 |
| Henry's Law Constant | 1.79 at 25 °C (Christie and Crisp, 1967) |
| Exposure limits | NIOSH REL: IDLH 200 ppm; OSHA PEL: TWA 25 ppm (100 mg/m3); ACGIH TLV: TWA 1 ppm, STEL 3 ppm (adopted). |
| Dielectric constant | 5.0(Ambient) |
| Stability | Stable. Extremely flammable. Readily forms explosive mixtures with air. Note low flash point. Incompatible with strong oxidizing agents, strong acids, ketones, aldehydes, halogenated hydrocarbons. |
| InChIKey | ZMANZCXQSJIPKH-UHFFFAOYSA-N |
| LogP | 1.65 |
| CAS DataBase Reference | 121-44-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Triethylamine(121-44-8) |
| EPA Substance Registry System | Triethylamine (121-44-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H302-H311+H331-H314-H335 | |||||||||
| Precautionary statements | P210-P280-P301+P312-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | F,C | |||||||||
| Risk Statements | 11-20/21/22-35 | |||||||||
| Safety Statements | 3-16-26-29-36/37/39-45-61 | |||||||||
| RIDADR | UN 1296 3/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | YE0175000 | |||||||||
| F | 34 | |||||||||
| Autoignition Temperature | 593 °F | |||||||||
| Hazard Note | Highly Flammable/Corrosive | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29211910 | |||||||||
| Toxicity | LD50 orally in rats: 0.46 g/kg (Smyth) | |||||||||
| IDLA | 200 ppm | |||||||||
| NFPA 704 |
|
Triethylamine price More Price(54)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | T0886 | Triethylamine ≥99% | 121-44-8 | 100ML | ₹3821.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W424601 | Triethylamine ≥99.5% | 121-44-8 | 1SAMPLE | ₹5141.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W424601 | Triethylamine ≥99.5% | 121-44-8 | 1KG | ₹7220.28 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T0886 | Triethylamine ≥99% | 121-44-8 | 500ML | ₹8270.3 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T0886 | Triethylamine ≥99% | 121-44-8 | 4X100ML | ₹11117.28 | 2022-06-14 | Buy |
Triethylamine Chemical Properties,Uses,Production
Chemical Properties
Triethylamine is a colorless to yellowish liquid with a strong ammonia to fish-like odor. It is a base commonly used in organic chemistry to prepare esters and amides from acyl chlorides. Like other tertiary amines,it catalyzes the formation of urethane foams and epoxy resins.
Physical properties
Clear, colorless to light yellow flammable liquid with a strong, penetrating, ammonia-like odor. Experimentally determined detection and recognition odor threshold concentrations were <400 μg/m3 (<100 ppbv) and 1.1 mg/m3 (270 ppbv), respectively (Hellman and Small, 1974). An odor threshold concentration of 0.032 ppbv was determined by a triangular odor bag method (Nagata and Takeuchi, 1990).
Uses
Triethylamine is a base used to prepare esters and amides from acyl chlorides as well as in the synthesis of quaternary ammonium compounds. It acts as a catalyst in the formation of urethane foams and epoxy resins, dehydrohalogeantion reactions, acid neutralizers for condensation reactions and Swern oxidations. It finds application in reverse phase high-performance liquid chromatography (HPLC) as a mobile-phase modifier. It is also used as an accelerator activator for rubber, as a propellant, as a corrosion inhibitor, as a curing and hardening agent for polymers and for the desalination of seawater. Furthermore, it is used in the automotive casting industry and the textile industry.
Production Methods
Triethylamine is prepared by a vapor phase reaction of ammonia with ethanol or reaction of N,N-diethylacetamide with lithium aluminum hydride (Windholz et al 1983). It may also be produced from ethyl chloride and ammonia under heat and pressure (Hawley 1981) or by vapor phase alkylation of ammonia with ethanol (HSDB 1988). U.S. production is estimated at greater than 22,000 tons in 1972 (HSDB 1988).
Definition
ChEBI: Triethylamine is a tertiary amine that is ammonia in which each hydrogen atom is substituted by an ethyl group.
Application
Triethylamine (TEA, Et3N) is an aliphatic amine. It is used to catalytic solvent in chemical synthesis; accelerator activators for rubber; wetting, penetrating, and waterproofing agents of quaternary ammonium types; curing and hardening of polymers (e.g., corebinding resins); corrosion inhibitor; propellant.
Triethylamine has been used during the synthesis of:
5′-dimethoxytrityl-5-(fur-2-yl)-2′-deoxyuridine
3′-(2-cyanoethyl)diisopropylphosphoramidite-5′-dimethoxytrityl-5-(fur-2-yl)-2′-deoxyuridine
polyethylenimine600-β-cyclodextrin (PEI600-β-CyD)
It may be used as a homogeneous catalyst for the preparation of glycerol dicarbonate, via transesterification reaction between glycerol and dimethyl carbonate (DMC).
General Description
Triethylamine appears as a clear colorless liquid with a strong ammonia to fish-like odor. Flash point 20°F. Vapors irritate the eyes and mucous membranes. Less dense (6.1 lb / gal) than water. Vapors heavier than air. Produces toxic oxides of nitrogen when burned.
Reactivity Profile
Triethylamine reacts violently with oxidizing agents. Reacts with Al and Zn. Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Health Hazard
Vapors irritate nose, throat, and lungs, causing coughing, choking, and difficult breathing. Contact with eyes causes severe burns. Clothing wet with chemical causes skin burns. Triethylamine may also be irritating to skin and mucous membranes (Windholz et al 1983).
Fire Hazard
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Industrial uses
Triethylamine is used as an anti-livering agent for urea- and melamine-based enamels and in the recovery of gelled paint vehicles (HSDB 1988). It is also used as a catalyst for polyurethane foams, a flux for copper soldering, and as a catalytic solvent in chemical synthesis (Hawley 1981). Triethylamine is used in accelerating activators for rubber; as a corrosion inhibitor for polymers; a propellant; wetting, penetrating, and waterproofing agent of quaternary ammonium compounds; in curing and hardening of polymers (i.e. core-binding resins); and as a catalyst for epoxy resins (Hamilton and Hardy, 1974).
Safety Profile
Moderately toxic by ingestion and skin contact. Mildly toxic by inhalation. Human systemic effects: visual field changes. Experimental reproductive effects. Mutation data reported. A skin and severe eye irritant. Can cause kidney and liver damage. A very dangerous fire hazard when exposed to heat, flame, or oxidizers. Explosive in the form of vapor when exposed to heat or flame. Complex with dinitrogen tetraoxide explodes below 0°C when undduted with solvent. Exothermic reaction with maleic anhydride above 150°C. Can react with oxidzing materials. Incompatible with N2O4. To fight fire, use CO2, dry chemical, alcohol foam. When heated to decomposition it emits toxic fumes of NOx.
Potential Exposure
Triethylamine is and aliphatic amine used as a solvent; corrosion inhibitor; in chemical synthesis; and accelerator activators; paint remover; base in methylene chloride or other chlorinated solvents. TEA is used to solubilize 2,4,5-T in water and serves as a selective extractant in the purification of antibiotics. It is used to manufacture quaternary ammonia compounds and octadecyloxymethyltriethylammonium chloride; an agent used in textile treatment.
Carcinogenicity
TEA was not mutagenic in bacterial assays, but it did cause aneuploidy and chromosome aberrations in rats.
Environmental Fate
Photolytic. Low et al. (1991) reported that the photooxidation of aqueous tertiary amine
solutions by UV light in the presence of titanium dioxide resulted in the formation of ammonium
and nitrate ions.
Chemical/Physical. Triethylamine reacted with NOx in the dark to form diethylnitrosamine. In
an outdoor chamber, photooxidation by natural sunlight yielded the following products:
diethylnitramine, diethylformamide, diethylacetamide, ethylacetamide, diethylhydroxylamine,
ozone, acetaldehyde, and peroxyacetyl nitrate (Pitts et al., 1978).
Metabolism
There have been few studies on the metabolism of industrially important aliphatic amines such as triethylamine. It is generally assumed that amines not normally present in the body are metabolized by monoamine oxidase and diamine oxidase (histaminase).
Ultimately ammonia is formed and will be converted to urea. The hydrogen peroxide formed is acted upon by catalase and the aldehyde formed is thought to be converted to the corresponding carboxylic acid by the action of aldehyde oxidase (Beard and Noe 1981).
Shipping
UN1296 Triethylamine, Hazard Class: 3; Labels: 3-Flammable liquid, 8-Corrosive material.
Purification Methods
Dry triethylamine with CaSO4, LiAlH4, Linde type 4A molecular sieves, CaH2, KOH, or K2CO3, then distil it, either alone or from BaO, sodium, P2O5 or CaH2. It has also been distilled from zinc dust, under nitrogen. To remove traces of primary and secondary amines, triethylamine has been refluxed with acetic anhydride, benzoic anhydride, phthalic anhydride, then distilled, refluxed with CaH2 (ammonia-free) or KOH (or dried with activated alumina), and again distilled. Another purification method involved refluxing for 2hours with p-toluenesulfonyl chloride, then distilling. Grovenstein and Williams [J Am Chem Soc 83 412 1961] treated triethylamine (500mL) with benzoyl chloride (30mL), filtered off the precipitate, and refluxed the liquid for 1hour with a further 30mL of benzoyl chloride. After cooling, the liquid was filtered, distilled, and allowed to stand for several hours with KOH pellets. It was then refluxed with, and distilled from, stirred molten potassium. Triethylamine has been converted to its hydrochloride (see brlow), crystallised from EtOH (to m 254o), then liberated with aqueous NaOH, dried with solid KOH and distilled from sodium under N2. [Beilstein 4 H 99, 4 I 348, 4 II 593, 4 III 194, 4 IV 322.]
Incompatibilities
A strong base. Violent reaction with strong acids; halogenated compounds; and strong oxidizers. Attacks some forms of plastics, rubber and coatings. Corrosive to aluminum, zinc, copper, and their alloys in the presence of moisture. Reaction with nitrosating agents (e.g., nitrites, nitrous gases, and nitrous acid) capable of releasing carcinogenic nitrosamines.
Waste Disposal
Controlled incineration (incinerator equipped with a scrubber or thermal unit to reduce nitrogen oxides emissions).
Triethylamine Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| oswal chemicals | +91-9173644055 +91-9173644055 | Gujarat, India | 66 | 58 | Inquiry |
| Manas Petro Chem | +91-9004912487 +91-9004912487 | Maharashtra, India | 17 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| BASF India Limited | +91-2262785600 +91-2262785600 | Maharashtra, India | 209 | 58 | Inquiry |
| AKASH PHARMA EXPORTS | +91-9388123451 +91-9846039283 | Kerela, India | 470 | 58 | Inquiry |
| RYZE CHEMIE | +91-9702966440 +91-9702966440 | Maharashtra, India | 335 | 58 | Inquiry |
| Avra Synthesis Pvt Ltd | +914027175796 | New Delhi, India | 1606 | 30 | Inquiry |
| Spectrochem Pvt., Ltd. | 91-22-22016968 | Maharashtra, India | 183 | 58 | Inquiry |
| Jadavji & Sons | 91-22-24033236 | Maharashtra, India | 31 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ANJI BIOSCIENCES | 58 |
| oswal chemicals | 58 |
| Manas Petro Chem | 58 |
| JSK Chemicals | 58 |
| BASF India Limited | 58 |
| AKASH PHARMA EXPORTS | 58 |
| RYZE CHEMIE | 58 |
| Avra Synthesis Pvt Ltd | 30 |
| Spectrochem Pvt., Ltd. | 58 |
| Jadavji & Sons | 58 |
Related articles
- The toxic effects of Triethylamine
- Triethylamine is a colourless, unpleasant-smelling, inflammable liquid. It is absorbed well after both ingestion and inhalatio....
- Nov 5,2019





